5.6: Enzyme Inhibition
- Page ID
- 234009
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Explain what an enzyme inhibitor is.
- Distinguish between reversible and irreversible inhibitors.
- Distinguish between competitive and noncompetitive inhibitors.
Previously, we noted that enzymes are inactivated at high temperatures and by changes in pH. These are nonspecific factors that would inactivate any enzyme. The activity of enzymes can also be regulated by more specific inhibitors. Many compounds are poisons because they bind covalently to particular enzymes or kinds of enzymes and inactivate them (Table \(\PageIndex{1}\)).
| Poison | Formula | Example of Enzyme Inhibited | Action |
|---|---|---|---|
| arsenate | \(\ce{AsO4^{3−}}\) | glyceraldehyde 3-phosphate dehydrogenase | substitutes for phosphate |
| iodoacetate | \(\ce{ICH2COO^{−}}\) | triose phosphate dehydrogenase | binds to cysteine \(\ce{SH}\) group |
| diisopropylfluoro-phosphate (DIFP; a nerve poison) | 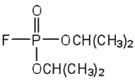 |
acetylcholinesterase | binds to serine \(\ce{OH}\) group |
Irreversible Inhibition: Poisons
An irreversible inhibitor inactivates an enzyme by bonding covalently to a particular group at the active site. The inhibitor-enzyme bond is so strong that the inhibition cannot be reversed by the addition of excess substrate. The nerve gases, especially Diisopropyl fluorophosphate (DIFP), irreversibly inhibit biological systems by forming an enzyme-inhibitor complex with a specific OH group of serine situated at the active sites of certain enzymes. The peptidases trypsin and chymotrypsin contain serine groups at the active site and are inhibited by DIFP.
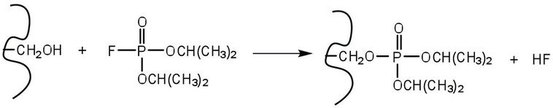
Reversible Inhibition
A reversible inhibitor inactivates an enzyme through noncovalent, more easily reversed, interactions. Unlike an irreversible inhibitor, a reversible inhibitor can dissociate from the enzyme. Reversible inhibitors include competitive inhibitors and noncompetitive inhibitors. (There are additional types of reversible inhibitors.) A competitive inhibitor is any compound that bears a structural resemblance to a particular substrate and thus competes with that substrate for binding at the active site of an enzyme. The inhibitor is not acted on by the enzyme but does prevent the substrate from approaching the active site.
Competitive vs Non-competitive inhibition. Image by CNX OpenStax, CC BY 4.0, via Wikimedia Commons
The degree to which a competitive inhibitor interferes with an enzyme’s activity depends on the relative concentrations of the substrate and the inhibitor. If the inhibitor is present in relatively large quantities, it will initially block most of the active sites. But because the binding is reversible, some substrate molecules will eventually bind to the active site and be converted to product. Increasing the substrate concentration promotes displacement of the inhibitor from the active site. Competitive inhibition can be completely reversed by adding substrate so that it reaches a much higher concentration than that of the inhibitor.
Studies of competitive inhibition have provided helpful information about certain enzyme-substrate complexes and the interactions of specific groups at the active sites. As a result, pharmaceutical companies have synthesized drugs that competitively inhibit metabolic processes in bacteria and certain cancer cells. Many drugs are competitive inhibitors of specific enzymes.
A classic example of competitive inhibition is the effect of malonate on the enzyme activity of succinate dehydrogenase (Figure \(\PageIndex{1}\)). Malonate and succinate are the anions of dicarboxylic acids and contain three and four carbon atoms, respectively. The malonate molecule binds to the active site because the spacing of its carboxyl groups is not greatly different from that of succinate. However, no catalytic reaction occurs because malonate does not have a CH2CH2 group to convert to CH=CH. This reaction will also be discussed in connection with the Krebs cycle and energy production.
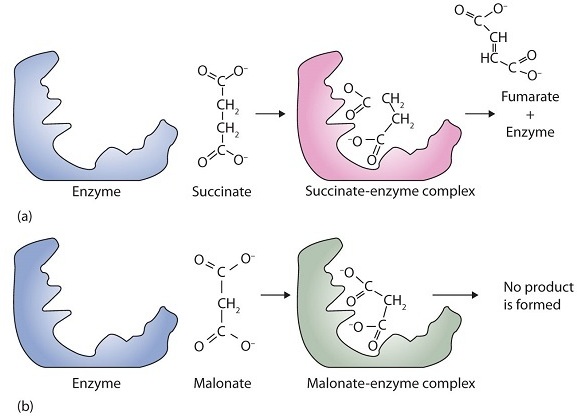
Some reversible inhibitors are noncompetitive. A noncompetitive inhibitor can combine with either the free enzyme or the enzyme-substrate complex because its binding site on the enzyme is distinct from the active site. This binging site is called the allosteric site (different from the active site) Binding of this kind of inhibitor alters the three-dimensional conformation of the enzyme, changing the configuration of the active site with one of two results. Either the enzyme-substrate complex does not form at its normal rate, or, once formed, it does not yield products at the normal rate. Because the inhibitor does not structurally resemble the substrate, the addition of excess substrate does not reverse the inhibitory effect.
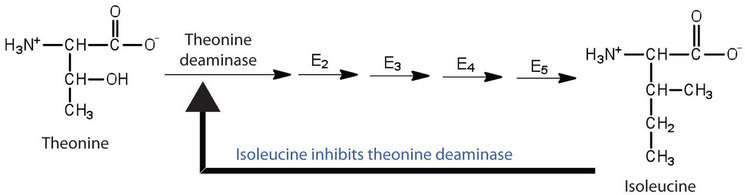
Feedback inhibition is a normal biochemical process that makes use of noncompetitive inhibitors to control some enzymatic activity. In this process, the final product inhibits the enzyme that catalyzes the first step in a series of reactions. Feedback inhibition is used to regulate the synthesis of many amino acids. For example, bacteria synthesize isoleucine from threonine in a series of five enzyme-catalyzed steps. As the concentration of isoleucine increases, some of it binds as a noncompetitive inhibitor to the first enzyme of the series (threonine deaminase), thus bringing about a decrease in the amount of isoleucine being formed (Figure \(\PageIndex{2}\)).
Summary
An irreversible inhibitor inactivates an enzyme by bonding covalently to a particular group at the active site. A reversible inhibitor inactivates an enzyme through noncovalent, reversible interactions. A competitive inhibitor competes with the substrate for binding at the active site of the enzyme. A noncompetitive inhibitor binds at a allosteric site distinct from the active site.
Concept Review Exercises
- What are the characteristics of an irreversible inhibitor?
- In what ways does a competitive inhibitor differ from a noncompetitive inhibitor?
Answers
- It inactivates an enzyme by bonding covalently to a particular group at the active site.
- A competitive inhibitor structurally resembles the substrate for a given enzyme and competes with the substrate for binding at the active site of the enzyme. A noncompetitive inhibitor binds at a site distinct from the active site and can bind to either the free enzyme or the enzyme-substrate complex.
Exercises
- What amino acid is present in the active site of all enzymes that are irreversibly inhibited by nerve gases such as DIFP?
- Oxaloacetate (OOCCH2COCOO) inhibits succinate dehydrogenase. Would you expect oxaloacetate to be a competitive or noncompetitive inhibitor? Explain.
Answer
-
serine


