1.3: Bonding in the Carbonyl Group
- Page ID
- 227525
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A carbonyl group is a chemically organic functional group composed of a carbon atom double-bonded to an oxygen atom --> [C=O] The simplest carbonyl groups are aldehydes and ketones usually attached to another carbon compound. These structures can be found in many aromatic compounds contributing to smell and taste.
Introduction
Before going into anything in depth be sure to understand that the C=O entity itself is known as the "Carbonyl group" while the members of this group are called "carbonyl compounds" --> X-C=O. The carbon and oxygen are usually sp2 hybridized and planar.
Carbonyl Group Double Bonds
The double bonds in alkenes and double bonds in carbonyl groups are VERY different in terms of reactivity. The C=C is less reactive due to C=O electronegativity attributed to the oxygen and its two lone pairs of electrons. One pair of the oxygen lone pairs are located in 2s while the other pair are in 2p orbital where its axis is directed perpendicular to the direction of the pi orbitals. The Carbonyl groups properties are directly tied to its electronic structure as well as geometric positioning. For example, the electronegativity of oxygen also polarizes the pi bond allowing the single bonded substituent connected to become electron withdrawing.
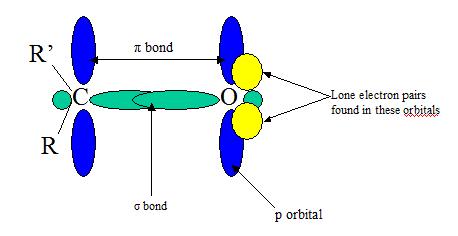
*Note: Both the pi bonds are in phase (top and botom blue ovals)
The double bond lengths of a carbonyl group is about 1.2 angstroms and the strength is about 176-179 kcal/mol). It is possible to correlate the length of a carbonyl bond with its polarity; the longer the bond meaing the lower the polarity. For example, the bond length in C=O is larger in acetaldehyde than in formaldehyde (this of course takes into account the inductive effect of CH3 in the compound).
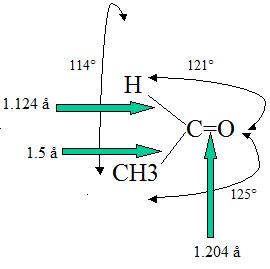
Polarization
As discussed before, we understand that oxygen has two lone pairs of electrons hanging around. These electrons make the oxygen more electronegative than carbon. The carbon is then partially postive (electrophillic) and the oxygen partially negative (nucleophillic). The polarizability is denoted by a lowercase delta and a positive or negative superscript depending. For example, carbon would have d+ and oxygen delta^(-). The polarization of carbonyl groups also effects the boiling point of aldehydes and ketones to be higher than those of hydrocarbons in the same amount. The larger the carbonyl compound the less soluble it is in water. If the compound exceeds six carbons it then becomes insoluble.
*For more information about carbonyl solubility, look in the "outside links" section
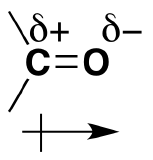
*Amides are the most stable of the carbonyl couplings due to the high-resonance stabilization between nitrogen-carbon and carbon-oxygen.
Some Carbonyl Compounds
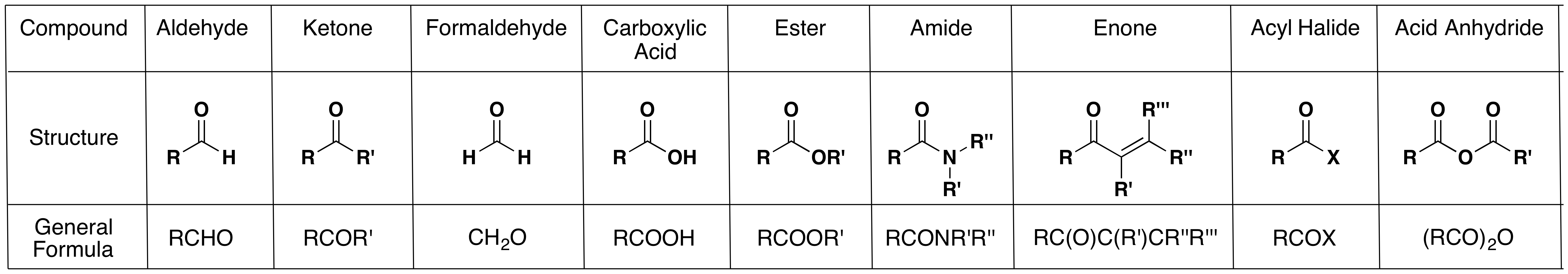
The carbonyl group is not only present in aldehydes and ketones. Other functional groups and compounds also contain a carbonyl group as part of their structures:
Nucleophile Addition to a Carbonyl Group
Just like alkenes with a C=C double bond, the carbonyl groups C=O is prone to additions reactions by nucleophillic attack. However, aldehydes and ketones are more reactive than alkenes because of carbon's partial positive charge and oxygen's partial negative charge (dipolar moment). The resonance of the carbon partial positive charge allows the negative charge on the nucleophile to attack the Carbonyl group and become a part of the structure and a positive charge (usually a proton hydrogen) attacks the oxygen. Just a reminder, the nucleophile is a good acid therefore "likes protons" so it will attack the side with a positive charge.
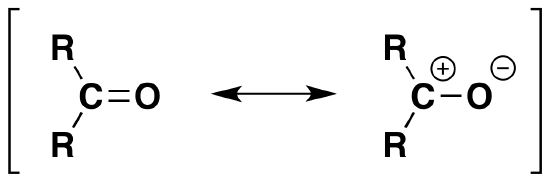
*Remember: due to the electronegative nature of oxygen the carbon is partially positive and oxygen is partially negative
Let´s imagine a carbonyl group reacting with a nucleophile Nu:

1 2 3
- The Nucleophile (Nu–) attacks the positively charged carbon and pushes one of the double bond electrons onto oxygen to give it a negative charge.
- The Nucleophile is now a part of the carbonyl structure with a negatively charged oxygen
- The negatively charged oxygen attacks the proton (H+) to give the resulting product above.
Problems
- What is the hybridization of the carbon in the C=O? the oxygen?
- Illustrate the correct partial positive/negative or polarization of a formaldehyde.
Answers
1. sp2;sp2
2. partial positive on the carbon and partial negative on the oxygen
References
- Patai, Saul, ed. The Chemistry of the Carbonyl Group. Vol. 1. London-New York-Sydney: Interscience, 1966.
- Zabicky, Jacob, ed. The Chemistry of the Carbonyl Group. Vol. 2. London-New York-Sydney: Interscience, 1966.
- Gutsche, C. David, author. Rinehart, Kenneth L., ed. The Chemistry of Carbonyl Groups. Vol.1 Endlewood Cliffs, New Jersey: Prentice-Hall, Inc., 1967
- Vollhardt, K. Peter C. "The Carbonyl Group (17.2)." Organic chemistry structure and function. 5th ed. Vol. 1. New York: W.H. Freeman, 2007.
Contributors
- Sharleen Agvateesiri

