4.E: Chemical Bonding and Molecular Geometry (Exercises)
- Page ID
- 221159
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)7.1: Ionic Bonding
Q7.1.1
Does a cation gain protons to form a positive charge or does it lose electrons?
S7.1.1
The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Positive charges form when electrons are lost.
Q7.1.2
Iron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and \(\ce{SO4^2-}\) ions. Explain why a sample of iron(III) sulfate is uncharged.
Q7.1.3
Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: P, I, Mg, Cl, In, Cs, O, Pb, Co?
S7.1.3
P, I, Cl, and O would form anions because they are nonmetals. Mg, In, Cs, Pb, and Co would form cations because they are metals.
Q7.1.4
Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: Br, Ca, Na, N, F, Al, Sn, S, Cd?
Q7.1.5
Predict the charge on the monatomic ions formed from the following atoms in binary ionic compounds:
- P
- Mg
- Al
- O
- Cl
- Cs
S7.1.5
P3–; Mg2+; Al3+; O2–; Cl–; Cs+
Q7.1.6
Predict the charge on the monatomic ions formed from the following atoms in binary ionic compounds:
- I
- Sr
- K
- N
- S
- In
S7.1.6
- I-
- Sr2+
- K+
- N3-
- S2-
- In3+
Q7.1.7
Write the electron configuration for each of the following ions:
- As3–
- I–
- Be2+
- Cd2+
- O2–
- Ga3+
- Li+
- (h) N3–
- (i) Sn2+
- (j) Co2+
- (k) Fe2+
- (l) As3+
S7.1.7
[Ar]4s23d104p6; [Kr]4d105s25p6 1s2 [Kr]4d10; [He]2s22p6; [Ar]3d10; 1s2 (h) [He]2s22p6 (i) [Kr]4d105s2 (j) [Ar]3d7 (k) [Ar]3d6, (l) [Ar]3d104s2
Q7.1.8
Write the electron configuration for the monatomic ions formed from the following elements (which form the greatest concentration of monatomic ions in seawater):
- Cl
- Na
- Mg
- Ca
- K
- Br
- Sr
- (h) F
Q7.1.9
Write out the full electron configuration for each of the following atoms and for the monatomic ion found in binary ionic compounds containing the element:
- Al
- Br
- Sr
- Li
- As
- S
S7.1.9
1s22s22p63s23p1; Al3+: 1s22s22p6; 1s22s22p63s23p63d104s24p5; 1s22s22p63s23p63d104s24p6; 1s22s22p63s23p63d104s24p65s2;
Sr2+: 1s22s22p63s23p63d104s24p6; 1s22s1;
Li+: 1s2; 1s22s22p63s23p63d104s24p3; 1s22s22p63s23p63d104s24p6; 1s22s22p63s23p4; 1s22s22p63s23p6
Q7.1.10
From the labels of several commercial products, prepare a list of six ionic compounds in the products. For each compound, write the formula. (You may need to look up some formulas in a suitable reference.)
7.3: Covalent Bonding
Why is it incorrect to speak of a molecule of solid NaCl?
NaCl consists of discrete ions arranged in a crystal lattice, not covalently bonded molecules.
What information can you use to predict whether a bond between two atoms is covalent or ionic?
Predict which of the following compounds are ionic and which are covalent, based on the location of their constituent atoms in the periodic table:
- Cl2CO
- MnO
- NCl3
- CoBr2
- K2S
- CO
- CaF2
- (h) HI
- (i) CaO
- (j) IBr
- (k) CO2
ionic: (b), (d), (e), (g), and (i); covalent: (a), (c), (f), (h), (j), and (k)
Explain the difference between a nonpolar covalent bond, a polar covalent bond, and an ionic bond.
From its position in the periodic table, determine which atom in each pair is more electronegative:
- Br or Cl
- N or O
- S or O
- P or S
- Si or N
- Ba or P
- N or K
Cl; O; O; S; N; P; N
From its position in the periodic table, determine which atom in each pair is more electronegative:
- N or P
- N or Ge
- S or F
- Cl or S
- H or C
- Se or P
- C or Si
From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
- C, F, H, N, O
- Br, Cl, F, H, I
- F, H, O, P, S
- Al, H, Na, O, P
- Ba, H, N, O, As
H, C, N, O, F; H, I, Br, Cl, F; H, P, S, O, F; Na, Al, H, P, O; Ba, H, As, N, O
From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
- As, H, N, P, Sb
- Cl, H, P, S, Si
- Br, Cl, Ge, H, Sr
- Ca, H, K, N, Si
- Cl, Cs, Ge, H, Sr
Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?
N, O, F, and Cl
Which is the most polar bond?
- C–C
- C–H
- N–H
- O–H
- Se–H
Identify the more polar bond in each of the following pairs of bonds:
- HF or HCl
- NO or CO
- SH or OH
- PCl or SCl
- CH or NH
- SO or PO
- CN or NN
HF; CO; OH; PCl; NH; PO; CN
Which of the following molecules or ions contain polar bonds?
- O3
- S8
- \(\ce{O2^2-}\)
- \(\ce{NO3-}\)
- CO2
- H2S
- \(\ce{BH4-}\)
7.4: Lewis Symbols and Structures
Q7.4.1
Write the Lewis symbols for each of the following ions:
- As3–
- I–
- Be2+
- O2–
- Ga3+
- Li+
- N3–
S7.4.1
eight electrons:
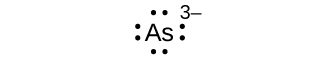
eight electrons:

no electrons
Be2+;
eight electrons:
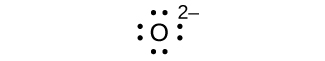
no electrons
Ga3+;
no electrons
Li+;
eight electrons:
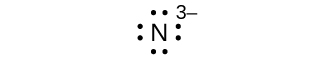
Q7.4.2
Many monatomic ions are found in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements:
- Cl
- Na
- Mg
- Ca
- K
- Br
- Sr
- F
Q7.4.3
Write the Lewis symbols of the ions in each of the following ionic compounds and the Lewis symbols of the atom from which they are formed:
- MgS
- Al2O3
- GaCl3
- K2O
- Li3N
- KF
(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)
 ;
;
(f)

In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element:
(a)

(b)

(c)

(d)

Write the Lewis structure for the diatomic molecule P2, an unstable form of phosphorus found in high-temperature phosphorus vapor.

Write Lewis structures for the following:
- H2
- HBr
- PCl3
- SF2
- H2CCH2
- HNNH
- H2CNH
- (h) NO–
- (i) N2
- (j) CO
- (k) CN–
Write Lewis structures for the following:
- O2
- H2CO
- AsF3
- ClNO
- SiCl4
- H3O+
- \(\ce{NH4+}\)
- (h) \(\ce{BF4-}\)
- (i) HCCH
- (j) ClCN
- (k) \(\ce{C2^2+}\)
(a)

In this case, the Lewis structure is inadequate to depict the fact that experimental studies have shown two unpaired electrons in each oxygen molecule.
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)
 ;
;
(f)
 ;
;
(g)
 ;
;
(h)
 ;
;
(i)
 ;
;
(j)
 ;
;
(k)

Write Lewis structures for the following:
- ClF3
- PCl5
- BF3
- \(\ce{PF6-}\)
Write Lewis structures for the following:
- SeF6
- XeF4
- \(\ce{SeCl3+}\)
- Cl2BBCl2 (contains a B–B bond)
SeF6:
 ;
;
XeF4:
 ;
;
\(\ce{SeCl3+}\):
 ;
;
Cl2BBCl2:

Write Lewis structures for:
- \(\ce{PO4^3-}\)
- \(\ce{ICl4-}\)
- \(\ce{SO3^2-}\)
- HONO
Correct the following statement: “The bonds in solid PbCl2 are ionic; the bond in a HCl molecule is covalent. Thus, all of the valence electrons in PbCl2 are located on the Cl– ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.”
Two valence electrons per Pb atom are transferred to Cl atoms; the resulting Pb2+ ion has a 6s2 valence shell configuration. Two of the valence electrons in the HCl molecule are shared, and the other six are located on the Cl atom as lone pairs of electrons.
Write Lewis structures for the following molecules or ions:
- SbH3
- XeF2
- Se8 (a cyclic molecule with a ring of eight Se atoms)
Methanol, H3COH, is used as the fuel in some race cars. Ethanol, C2H5OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO2 and H2O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.

Many planets in our solar system contain organic chemicals including methane (CH4) and traces of ethylene (C2H4), ethane (C2H6), propyne (H3CCCH), and diacetylene (HCCCCH). Write the Lewis structures for each of these molecules.
Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, Cl2CO. Write the Lewis structures for carbon tetrachloride and phosgene.

Identify the atoms that correspond to each of the following electron configurations. Then, write the Lewis symbol for the common ion formed from each atom:
- 1s22s22p5
- 1s22s22p63s2
- 1s22s22p63s23p64s23d10
- 1s22s22p63s23p64s23d104p4
- 1s22s22p63s23p64s23d104p1
The arrangement of atoms in several biologically important molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms.
the amino acid serine:

urea:

pyruvic acid:

uracil:

carbonic acid:

(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)

A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.
A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.

Two arrangements of atoms are possible for a compound with a molar mass of about 45 g/mol that contains 52.2% C, 13.1% H, and 34.7% O by mass. Write the Lewis structures for the two molecules.
How are single, double, and triple bonds similar? How do they differ?
Each bond includes a sharing of electrons between atoms. Two electrons are shared in a single bond; four electrons are shared in a double bond; and six electrons are shared in a triple bond.
7.5: Formal Charges and Resonance
Write resonance forms that describe the distribution of electrons in each of these molecules or ions.
- selenium dioxide, OSeO
- nitrate ion, \(\ce{NO3-}\)
- nitric acid, HNO3 (N is bonded to an OH group and two O atoms)
- benzene, C6H6:

the formate ion:

Write resonance forms that describe the distribution of electrons in each of these molecules or ions.
- sulfur dioxide, SO2
- carbonate ion, \(\ce{CO3^2-}\)
- hydrogen carbonate ion, \(\ce{HCO3-}\) (C is bonded to an OH group and two O atoms)
- pyridine:

the allyl ion:

(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)

Write the resonance forms of ozone, O3, the component of the upper atmosphere that protects the Earth from ultraviolet radiation.
Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. Write the resonance forms of the nitrite ion, \(\ce{NO2-}\).

In terms of the bonds present, explain why acetic acid, CH3CO2H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid, only contains one type of carbon-oxygen bond. The skeleton structures of these species are shown:

Write the Lewis structures for the following, and include resonance structures where appropriate. Indicate which has the strongest carbon-oxygen bond.
- CO2
- CO
(a)

(b)

CO has the strongest carbon-oxygen bond because there is a triple bond joining C and O. CO2 has double bonds.
Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate.
Determine the formal charge of each element in the following:
- HCl
- CF4
- PCl3
- PF5
H: 0, Cl: 0; C: 0, F: 0; P: 0, Cl 0; P: 0, F: 0
Determine the formal charge of each element in the following:
- H3O+
- \(\ce{SO4^2-}\)
- NH3
- \(\ce{O2^2-}\)
- H2O2
Calculate the formal charge of chlorine in the molecules Cl2, BeCl2, and ClF5.
Cl in Cl2: 0; Cl in BeCl2: 0; Cl in ClF5: 0
Calculate the formal charge of each element in the following compounds and ions:
- F2CO
- NO–
- \(\ce{BF4-}\)
- \(\ce{SnCl3-}\)
- H2CCH2
- ClF3
- SeF6
- (h) \(\ce{PO4^3-}\)
Draw all possible resonance structures for each of these compounds. Determine the formal charge on each atom in each of the resonance structures:
- O3
- SO2
- \(\ce{NO2-}\)
- \(\ce{NO3-}\)
 ;
;
(b)
 ;
;
(c)
![[Two Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom with one lone pair of electrons single bonded to an oxygen atom with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure appears as a mirror image of the left and the symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis.]](http://cnx.org/resources/1fdade826754e56f88dd8009bac80fa91af0bebc/CNX_Chem_07_04_Exercis12c_img.jpg) ;
;
(d)
![[Three Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The single bonded oxygen atoms are labeled, from the top of the structure and going clockwise, “open parenthesis, negative 1, close parenthesis, open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The middle structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons, one of which is labeled “open parenthesis, positive 1, close parenthesis” and double bonded to an oxygen atom with two lone pairs of electrons labeled “open parenthesis, 0, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. One of the single bonded oxygen atoms is labeled, “open parenthesis, negative 1, close parenthesis while the double bonded oxygen is labeled, “open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis” and “open parenthesis, 0, close parenthesis”.]](http://cnx.org/resources/263fd2cd3c8d574474c489b5bb2f41b94cd81ae5/CNX_Chem_07_04_Exercis12d_img.jpg)
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in nitrosyl chloride: ClNO or ClON?
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in hypochlorous acid: HOCl or OClH?
HOCl
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in sulfur dioxide: OSO or SOO?
Draw the structure of hydroxylamine, H3NO, and assign formal charges; look up the structure. Is the actual structure consistent with the formal charges?
The structure that gives zero formal charges is consistent with the actual structure:

Iodine forms a series of fluorides (listed here). Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
- IF
- IF3
- IF5
- IF7
Write the Lewis structure and chemical formula of the compound with a molar mass of about 70 g/mol that contains 19.7% nitrogen and 80.3% fluorine by mass, and determine the formal charge of the atoms in this compound.
NF3;

Which of the following structures would we expect for nitrous acid? Determine the formal charges:

Sulfuric acid is the industrial chemical produced in greatest quantity worldwide. About 90 billion pounds are produced each year in the United States alone. Write the Lewis structure for sulfuric acid, H2SO4, which has two oxygen atoms and two OH groups bonded to the sulfur.

7.6: Strengths of Ionic and Covalent Bonds
Which bond in each of the following pairs of bonds is the strongest?
- C–C or \(\mathrm{C=C}\)
- C–N or \(\mathrm{C≡N}\)
- \(\mathrm{C≡O}\) or \(\mathrm{C=O}\)
- H–F or H–Cl
- C–H or O–H
- C–N or C–O
Using the bond energies in Table, determine the approximate enthalpy change for each of the following reactions:
- \(\ce{H2}(g)+\ce{Br2}(g)⟶\ce{2HBr}(g)\)
- \(\ce{CH4}(g)+\ce{I2}(g)⟶\ce{CH3I}(g)+\ce{HI}(g)\)
- (c) \(\ce{C2H4}(g)+\ce{3O2}(g)⟶\ce{2CO2}(g)+\ce{2H2O}(g)\)
- −114 kJ;
- 30 kJ;
- (c) −1055 kJ
Using the bond energies in Table, determine the approximate enthalpy change for each of the following reactions:
- \(\ce{Cl2}(g)+\ce{3F2}(g)⟶\ce{2ClF3}(g)\)
- \(\mathrm{H_2C=CH_2}(g)+\ce{H2}(g)⟶\ce{H3CCH3}(g)\)
- (c) \(\ce{2C2H6}(g)+\ce{7O2}(g)⟶\ce{4CO2}(g)+\ce{6H2O}(g)\)
When a molecule can form two different structures, the structure with the stronger bonds is usually the more stable form. Use bond energies to predict the correct structure of the hydroxylamine molecule:

The greater bond energy is in the figure on the left. It is the more stable form.
How does the bond energy of HCldiffer from the standard enthalpy of formation of HCl(g)?
Using the standard enthalpy of formation data in Appendix G, show how the standard enthalpy of formation of HCl(g) can be used to determine the bond energy.
\(\ce{HCl}(g)⟶\dfrac{1}{2}\ce{H2}(g)+\dfrac{1}{2}\ce{Cl2}(g)\hspace{20px}ΔH^\circ_1=−ΔH^\circ_{\ce f[\ce{HCl}(g)]}\\
\dfrac{1}{2}\ce{H2}(g)⟶\ce{H}(g)\hspace{105px}ΔH^\circ_2=ΔH^\circ_{\ce f[\ce H(g)]}\\
\underline{\dfrac{1}{2}\ce{Cl2}(g)⟶\ce{Cl}(g)\hspace{99px}ΔH^\circ_3=ΔH^\circ_{\ce f[\ce{Cl}(g)]}}\\
\ce{HCl}(g)⟶\ce{H}(g)+\ce{Cl}(g)\hspace{58px}ΔH^\circ_{298}=ΔH^\circ_1+ΔH^\circ_2+ΔH^\circ_3\)
\(\begin{align}
D_\ce{HCl}=ΔH^\circ_{298}&=ΔH^\circ_{\ce f[\ce{HCl}(g)]}+ΔH^\circ_{\ce f[\ce H(g)]}+ΔH^\circ_{\ce f[\ce{Cl}(g)]}\\
&=\mathrm{−(−92.307\:kJ)+217.97\:kJ+121.3\:kJ}\\
&=\mathrm{431.6\:kJ}
\end{align}\)
Using the standard enthalpy of formation data in Appendix G, calculate the bond energy of the carbon-sulfur double bond in CS2.
Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the S–F bond in SF4(g) or in SF6(g)?
The S–F bond in SF4 is stronger.
Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the P–Cl bond in PCl3(g) or in PCl5(g)?
Complete the following Lewis structure by adding bonds (not atoms), and then indicate the longest bond:


The C–C single bonds are longest.
Use the bond energy to calculate an approximate value of ΔH for the following reaction. Which is the more stable form of FNO2?

Use principles of atomic structure to answer each of the following:1
- The radius of the Ca atom is 197 pm; the radius of the Ca2+ ion is 99 pm. Account for the difference.
- The lattice energy of CaO(s) is –3460 kJ/mol; the lattice energy of K2O is –2240 kJ/mol. Account for the difference.
- (c) Given these ionization values, explain the difference between Ca and K with regard to their first and second ionization energies.
| Element | First Ionization Energy (kJ/mol) | Second Ionization Energy (kJ/mol) |
|---|---|---|
| K | 419 | 3050 |
| Ca | 590 | 1140 |
The first ionization energy of Mg is 738 kJ/mol and that of Al is 578 kJ/mol. Account for this difference.
When two electrons are removed from the valence shell, the Ca radius loses the outermost energy level and reverts to the lower n = 3 level, which is much smaller in radius. The +2 charge on calcium pulls the oxygen much closer compared with K, thereby increasing the lattice energy relative to a less charged ion. (c) Removal of the 4s electron in Ca requires more energy than removal of the 4s electron in K because of the stronger attraction of the nucleus and the extra energy required to break the pairing of the electrons. The second ionization energy for K requires that an electron be removed from a lower energy level, where the attraction is much stronger from the nucleus for the electron. In addition, energy is required to unpair two electrons in a full orbital. For Ca, the second ionization potential requires removing only a lone electron in the exposed outer energy level. In Al, the removed electron is relatively unprotected and unpaired in a p orbital. The higher energy for Mg mainly reflects the unpairing of the 2s electron.
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 200.8 pm. NaF crystallizes in the same structure as LiF but with a Na–F distance of 231 pm. Which of the following values most closely approximates the lattice energy of NaF: 510, 890, 1023, 1175, or 4090 kJ/mol? Explain your choice.
For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
- MgO
- SrO
- (c) KF
- CsF
- MgF2
(d)
The reaction of a metal, M, with a halogen, X2, proceeds by an exothermic reaction as indicated by this equation: \(\ce{M}(s)+\ce{X2}(g)⟶\ce{MX2}(s)\). For each of the following, indicate which option will make the reaction more exothermic. Explain your answers.
- a large radius vs. a small radius for M+2
- a high ionization energy vs. a low ionization energy for M
- (c) an increasing bond energy for the halogen
- a decreasing electron affinity for the halogen
- an increasing size of the anion formed by the halogen
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4008 kJ/mol? Explain your choice.
4008 kJ/mol; both ions in MgO have twice the charge of the ions in LiF; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
Which compound in each of the following pairs has the larger lattice energy? Note: Mg2+ and Li+ have similar radii; O2– and F– have similar radii. Explain your choices.
- MgO or MgSe
- LiF or MgO
- (c) Li2O or LiCl
- Li2Se or MgO
Which compound in each of the following pairs has the larger lattice energy? Note: Ba2+ and
K+ have similar radii; S2– and Cl– have similar radii. Explain your choices.
- K2O or Na2O
- K2S or BaS
- (c) KCl or BaS
- BaS or BaCl2
Na2O; Na+ has a smaller radius than K+; BaS; Ba has a larger charge than K; (c) BaS; Ba and S have larger charges; BaS; S has a larger charge
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- MgO
- SrO
- (c) KF
- CsF
- MgF2
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- K2S
- K2O
- (c) CaS
- Cs2S
- CaO
(e)
The lattice energy of KF is 794 kJ/mol, and the interionic distance is 269 pm. The Na–F
distance in NaF, which has the same structure as KF, is 231 pm. Which of the following values is the closest approximation of the lattice energy of NaF: 682 kJ/mol, 794 kJ/mol, 924 kJ/mol, 1588 kJ/mol, or 3175 kJ/mol? Explain your answer.
7.7: Molecular Structure and Polarity
Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is bent. The HBeH molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
What feature of a Lewis structure can be used to tell if a molecule’s (or ion’s) electron-pair geometry and molecular structure will be identical?
Explain the difference between electron-pair geometry and molecular structure.
Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
Why is the H–N–H angle in NH3 smaller than the H–C–H bond angle in CH4? Why is the H–N–H angle in \(\ce{NH4+}\) identical to the H–C–H bond angle in CH4?
Explain how a molecule that contains polar bonds can be nonpolar.
As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar.
As a general rule, MXn molecules (where M represents a central atom and X represents terminal atoms; n = 2 – 5) are polar if there is one or more lone pairs of electrons on M. NH3 (M = N, X = H, n = 3) is an example. There are two molecular structures with lone pairs that are exceptions to this rule. What are they?
Predict the electron pair geometry and the molecular structure of each of the following molecules or ions:
- SF6
- PCl5
- (c) BeH2
- \(\ce{CH3+}\)
- Both the electron geometry and the molecular structure are octahedral.
- Both the electron geometry and the molecular structure are trigonal bipyramid.
- (c) Both the electron geometry and the molecular structure are linear.
- Both the electron geometry and the molecular structure are trigonal planar.
Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
- \(\ce{IF6+}\)
- CF4
- (c) BF3
- \(\ce{SiF5-}\)
- BeCl2
What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?
- ClF5
- \(\ce{ClO2-}\)
- (c) \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
electron-pair geometry: octahedral, molecular structure: square pyramidal; electron-pair geometry: tetrahedral, molecular structure: bent; (c) electron-pair geometry: octahedral, molecular structure: square planar; electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; electron-pair geometry: trigonal bypyramidal, molecular structure: seesaw; electron-pair geometry: tetrahedral, molecular structure: bent (109°)
Predict the electron pair geometry and the molecular structure of each of the following ions:
- H3O+
- \(\ce{PCl4-}\)
- (c) \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- (g) SF2
Identify the electron pair geometry and the molecular structure of each of the following molecules:
- ClNO (N is the central atom)
- CS2
- (c) Cl2CO (C is the central atom)
- Cl2SO (S is the central atom)
- SO2F2 (S is the central atom)
- XeO2F2 (Xe is the central atom)
- (g) \(\ce{ClOF2+}\) (Cl is the central atom)
electron-pair geometry: trigonal planar, molecular structure: bent (120°); electron-pair geometry: linear, molecular structure: linear; (c) electron-pair geometry: trigonal planar, molecular structure: trigonal planar; electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; electron-pair geometry: tetrahedral, molecular structure: tetrahedral; electron-pair geometry: trigonal bipyramidal, molecular structure: seesaw; (g) electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
Predict the electron pair geometry and the molecular structure of each of the following:
- IOF5 (I is the central atom)
- POCl3 (P is the central atom)
- (c) Cl2SeO (Se is the central atom)
- ClSO+ (S is the central atom)
- F2SO (S is the central atom)
- \(\ce{NO2-}\)
- (g) \(\ce{SiO4^4-}\)
Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- ClF5
- \(\ce{ClO2-}\)
- (c) \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
- (g) XeF2
All of these molecules and ions contain polar bonds. Only ClF5, \(\ce{ClO2-}\), PCl3, SeF4, and \(\ce{PH2-}\) have dipole moments.
Which of the molecules and ions in Exercise contain polar bonds? Which of these molecules and ions have dipole moments?
- H3O+
- \(\ce{PCl4-}\)
- (c) \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- (g) SF2
Which of the following molecules have dipole moments?
- CS2
- SeS2
- (c) CCl2F2
- PCl3 (P is the central atom)
- ClNO (N is the central atom)
SeS2, CCl2F2, PCl3, and ClNO all have dipole moments.
Identify the molecules with a dipole moment:
- SF4
- CF4
- (c) Cl2CCBr2
- CH3Cl
- H2CO
The molecule XF3 has a dipole moment. Is X boron or phosphorus?
P
The molecule XCl2 has a dipole moment. Is X beryllium or sulfur?
Is the Cl2BBCl2 molecule polar or nonpolar?
nonpolar
There are three possible structures for PCl2F3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.
Describe the molecular structure around the indicated atom or atoms:
- the sulfur atom in sulfuric acid, H2SO4 [(HO)2SO2]
- the chlorine atom in chloric acid, HClO3 [HOClO2]
- (c) the oxygen atom in hydrogen peroxide, HOOH
- the nitrogen atom in nitric acid, HNO3 [HONO2]
- the oxygen atom in the OH group in nitric acid, HNO3 [HONO2]
- the central oxygen atom in the ozone molecule, O3
- (g) each of the carbon atoms in propyne, CH3CCH
- (h) the carbon atom in Freon, CCl2F2
- (i) each of the carbon atoms in allene, H2CCCH2
tetrahedral; trigonal pyramidal; (c) bent (109°); trigonal planar; bent (109°); bent (109°); (g) CH3CCH tetrahedral, CH3CCH linear; (h) tetrahedral; (i) H2CCCH2 linear; H2CCCH2 trigonal planar
Draw the Lewis structures and predict the shape of each compound or ion:
- CO2
- \(\ce{NO2-}\)
- (c) SO3
- \(\ce{SO3^2-}\)
A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.

A molecule with the formula AB3, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion that has each shape.
Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:
- \(\ce{CS3^2-}\)
- CS2
- (c) CS
predict the molecular shapes for \(\ce{CS3^2-}\) and CS2 and explain how you arrived at your predictions
(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
\(\ce{CS3^2-}\) includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar; CS2 has only two regions of electron density (all bonds with no lone pairs); the shape is linear
What is the molecular structure of the stable form of FNO2? (N is the central atom.)
A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen. What is its molecular structure?
The Lewis structure is made from three units, but the atoms must be rearranged:

Use the simulation to perform the following exercises for a two-atom molecule:
- Adjust the electronegativity value so the bond dipole is pointing toward B. Then determine what the electronegativity values must be to switch the dipole so that it points toward A.
- With a partial positive charge on A, turn on the electric field and describe what happens.
- (c) With a small partial negative charge on A, turn on the electric field and describe what happens.
- Reset all, and then with a large partial negative charge on A, turn on the electric field and describe what happens.
Use the simulation to perform the following exercises for a real molecule. You may need to rotate the molecules in three dimensions to see certain dipoles.
- Sketch the bond dipoles and molecular dipole (if any) for O3. Explain your observations.
- Look at the bond dipoles for NH3. Use these dipoles to predict whether N or H is more electronegative.
- (c) Predict whether there should be a molecular dipole for NH3 and, if so, in which direction it will point. Check the molecular dipole box to test your hypothesis.
The molecular dipole points away from the hydrogen atoms.
Use the Molecule Shape simulator to build a molecule. Starting with the central atom, click on the double bond to add one double bond. Then add one single bond and one lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular structure and predict the bond angle. Then click the check boxes at the bottom and right of the simulator to check your answers.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select H2O. Switch between the “real” and “model” modes. Explain the difference observed.
The structures are very similar. In the model mode, each electron group occupies the same amount of space, so the bond angle is shown as 109.5°. In the “real” mode, the lone pairs are larger, causing the hydrogens to be compressed. This leads to the smaller angle of 104.5°.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select “model” mode and S2O. What is the model bond angle? Explain whether the “real” bond angle should be larger or smaller than the ideal model angle.


