Extraction of Caffeine From Tea Leaves
- Page ID
- 459412
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Objective: To isolate caffeine from tea leaves and to obtain supporting evidence regarding its identity and purity.
Background. In last week’s lab you separated the components of a commercial pain medication using thin-layer chromatography. This technique is very useful for identifying compounds, as well as for determining the number of major components there are in a mixture. What it isn’t good for, however, is isolating useful quantities of materials from mixtures. For example, the mass of the compounds that you applied to the TLC plates were on the order of micrograms (10-6 grams). Eden if you could remove the compound from the silica gel support material, there would be far too little of it to do anything practical. Therefore, if you have a mixture from which you want to isolate a particular compound, TLC would not be an effective method but, fortunately, other separation techniques are.
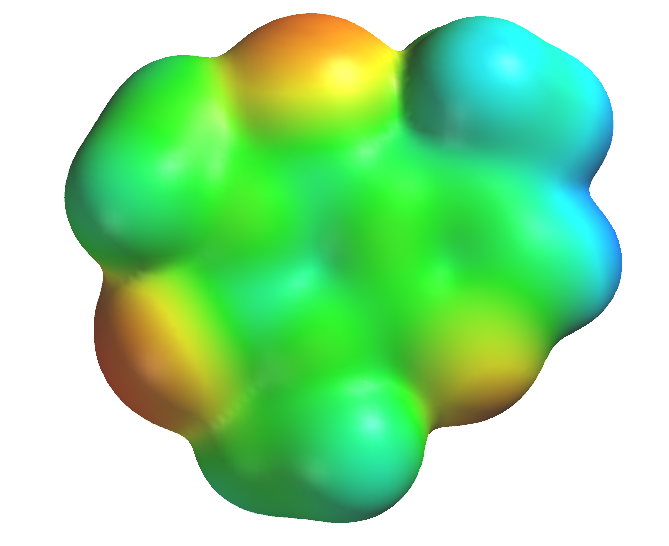
Today’s lab introduces you to one of those: extraction. In it, you will use a relatively nonpolar solvent, dichloromethane, CH2Cl2, to selectively remove caffeine from a sample of tea you will brew. Caffeine, the line diagram and esp map of which are shown below, is a stimulant that is found in tea, coffee, chocolate and is often included in pain medication (as you saw last week!) to counteract some of the depressant effects of the active pain relievers. The esp map indicates that it is somewhat polar, having regions of positive (blue) and negative (red) electric charges on its surface. These charges help explain why caffeine had such a low Rf value on the TLC results you saw last week; the charged portions of the molecule are strongly attracted to the charged particles on the TLC plate and resist moving with the eluent.
 |
 |
Because caffeine is somewhat polar, it dissolves in water to a limited extent, especially hot water. But it is even more soluble in dichloromethane, a less polar solvent that is not miscible with water. This provides the basis for this laboratory. You will brew tea and the boiling water will remove the caffeine from the leaves. You will then extract the caffeine into dichloromethane as explained below.
Tea leaves are a complex mixture of a wide range of materials. Cellulose provides the bulk of the leaf structure and gives support to the individual cells which contain a range of proteins, lipids, sugars, and pigments as well as salts, minerals and other compounds, including caffeine. Only a small fraction of the total mass is soluble in water, thus the tea bags will still contain the bulk of the leaves after you finish brewing. Of the compounds that are dissolved in the boiling water, most are not also soluble in nonpolar solvents, but a few are, such as caffeine, tannins, and gallic acid. To prevent the latter compounds from contaminating your caffeine extract, you will add sodium carbonate (Na2CO3) to your brewed tea while it is still hot. This reacts with tannins and gallic acid to form products that are negatively charged and, as a result, have dramatically reduced solubility in dichloromethane. Extraction of this tea mixture into dichloromethane using a separatory funnel should yield nearly pure caffeine.
After performing the extraction you should have a clear, colorless solution that consists of caffeine, the solute, and dichloromethane, the solvent. At this point you will perform a TLC on your extract and compare against a sample of pure caffeine using TLC. The Rf values should match and provide evidence that you successfully extracted the caffeine from the tea leaves (but it doesn’t prove it - why not?). It will also reveal if you have any significant impurities (how so?). To end the lab, you will place your sample of caffeine extract in a fume hood (covered with a watch glass) and allow the solvent to evaporate over the next week. When you come back to the lab next time, carefully weigh the sample and estimate the percent caffeine in the tea leaves (each tea bag has approximately 2.3 g of dried tea leaves). Describe what the material looks like physically and compare your observations to descriptions available elsewhere.
Procedure for Caffeine Extraction (to be performed in pairs)
-
Place about 100 mL of deionized water into a beaker (250 mL) and bring to a gentle boil on a hot plate.
-
After boiling for 15 minutes, carefully remove the tea bags and place them in a clean beaker to cool off.
-
While the tea bags are cooling add 2.5 grams of sodium carbonate (Na2CO3) to the tea extract and stir until dissolved.
-
When the tea bags have cooled, squeeze the liquid out of them and add the liquid to the rest of the tea/sodium carbonate mixture; the tea bags can be thrown out in the regular trash at this point.
-
Place the tea extract in an ice bath and allow it to cool down to room temperature (or below)
-
As demonstrated in lab, add the tea to a 250 mL separatory funnel (with the stopcock closed!); add 20 mL of dichloromethane (hazard!).
-
Place a stopper on the top of the separatory funnel and gently agitate the mixture as demonstrated in lab.
-
Allow the two layers to separate and then drain the bottom layer into a clean beaker, being careful to exclude any of the aqueous layer or any emulsified mixture; the solution should be clear and free from much color.
-
Repeat steps 7 and 8 twice with two additional portions of dichloromethane, draining them into the same beaker; the total volume of dichloromethane solution should be roughly 60 mL.
-
Spot a TLC plate with your solution as well as a solution of pure caffeine; develop using 100% ethyl acetate.
-
Place the beaker containing the caffeine in a fume hood with a watch glass over it; allow the solvent to evaporate (this will take a few days)

