4.7: Resonance and Formal Charge, Or, What To Do When the "Rules" Don't Work
- Page ID
- 419685
This page is a draft and is under active development.
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There is no denying the usefulness of the concepts presented in the previous section. Armed only with a few foundational ideas, you can draw electron dot diagrams for literally thousands of molecules and estimate, to within a couple of degrees, their bond angles as well as a get a sense of their relative bond lengths. That's an astounding amount of insight given the simplicity of the concepts involved. This is not say, however, that all predictions based on these ideas will be accurate. There are cases where the principles, as laid out thus far, will lead to predictions that don't agree well with observed structures. The good news is that there is one particular "red flag" to be on the lookout for that will let you know when your predictions will be in error and – just as importantly – lets you know when to modify your approach to improve their accuracy. To illustrate, we'll construct the electron dot diagram for ozone, O3, the allotrope of oxygen we used in Table 4-3 to illustrate the molecular geometry of atoms with a steric number of 3 and 1 lone pair. We provided an electron dot diagram at the time but it may not be have been clear how it was generated.
We'll beginning in the usual way, with the individual atoms (Figure 4-33). It is easy to see how the central oxygen atom can bond to the other two, thereby satisfying its own octet. But we run into a problem at that point, however. The resulting diagram leaves both terminal oxygen atoms with only seven electrons. How can these terminal atoms achieve their octets? We can shuffle the electrons around as follows:
a) remove one of the unpaired electrons from one terminal atom and placing it on the other (this completes the octet of the latter and leaves the former with only 6 electrons);
b) use one of the lone pairs on the central atom to form a double bond to the electron deficient atom, thereby satisfying the latter's octet without comprising the former's.
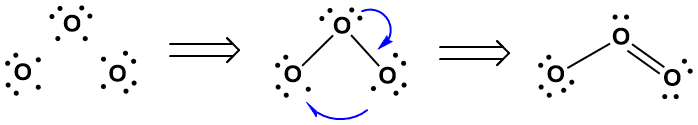 Figure 4-33. "Building" Ozone. The electron dot structure for ozone is depicted by first making single bonds between a central oxygen atom and two terminal atoms, then moving one of the unpaired electrons from one terminal atom to the other (shown with the lower arrow) and making a double bond to complete the octet of the electron deficient atoms (shown with the upper arrow). A note on conventions for future reference: the full arrowhead used in the upper arrow indicates that two electrons are moving, while the fishhook, or half arrowhead, indicates that only one electron is moving.
Figure 4-33. "Building" Ozone. The electron dot structure for ozone is depicted by first making single bonds between a central oxygen atom and two terminal atoms, then moving one of the unpaired electrons from one terminal atom to the other (shown with the lower arrow) and making a double bond to complete the octet of the electron deficient atoms (shown with the upper arrow). A note on conventions for future reference: the full arrowhead used in the upper arrow indicates that two electrons are moving, while the fishhook, or half arrowhead, indicates that only one electron is moving.
The above may strike you as being too clever by half [25]. But electrons really can move around in structures in ways that might seem counterintuitive. To anthropormorphosize a bit, they lack any sort of loyalty to the atoms to which they were originally associated and can move within molecules if it results in greater stability (i.e., minimizes the potential energy). The above electron rearrangement, even if it strikes you as "cheating" in some ways, ultimately gets us to a reasonable place in that the octets of all the atoms are satisfied. Now, let's look at the structural implications of the electron dot diagram, namely the bond angles and bond lengths that it predicts. Based on the ideas we laid out in the previous section, ozone should:
- have an O-O-O bond angle of 120° because the steric number of the central oxygen is 3 and it has one lone pair; and
- have two oxygen-oxygen bonds of different lengths; this is because the electron dot structure has one single bond and one double bond. We can estimate what these lengths will be by looking at related structures: the oxygen-oxygen double bond of O2 is 121 pm in length, while that of oxygen-oxygen single bonds average about 143 pm. We should expect to see bond lengths similar to these if our model of ozone is accurate.
We can now evaluate the accuracy of the predictions. First, the observed bond angle is 117°, which agrees fairly well with the VSEPR prediction. And the bond lengths? "Houston, we have a problem." We run into a major discrepancy between prediction and observation at this point: the oxygen-oxygen bond lengths of ozone are identical! Both are 128 pm, in between the two different bond lengths we expected to see. This is telling us something important: the bonding predicted by the electron dot structure is wrong. Why is this? And how can we fix it?
The problem actually originates with how we defined a covalent bond in the first place. Up until now, we've limited ourselves to the idea that bonds consist of two electrons (or multiples of two) shared between two atoms. While this works in many cases, it is actually an oversimplified view. Nature simply doesn't lend itself to rigorously following such simple formulations. It turns out that electrons are more "fluid" than our simple approach implies and covalent bonds can link more than two atoms at the same time. In other words, there is nothing inherent in the properties of electrons that limits them to staying localized between only two adjacent atoms. We need to employ a more sophisticated approach to bonding, but that doesn't mean we have to completely abandon the approach we've used thus far – which, as we said, is quite powerful and broadly applicable to many different molecules.
To refine the model of ozone we developed above, we should recognize that we made one completely arbitrary decision in constructing its electron dot diagram. Specifically, we took one electron from the rightmost oxygen in Figure 4-33 and placed it on the right left. This, in turn, forced us to place the double bond between the central oxygen and the one on the right. You can probably see that we could just as easily have done the opposite: moving an electron from the left to the right, and then placing the double bond on the left. The arbitrariness of that decision is is the red flag we mentioned at the outset of this section. To wit: when the placement of multiple bonds and/or lone pairs is arbitrary, the resulting electron dot structure will not provide an accurate depiction of the actual bonding in the molecule or polyatomic ion. To get a more accurate picture of the bonding you need to consider all the possible structures simultaneously. For example, there are two possible structures one could draw for ozone, labeled A and B, below (Figure 4-34).
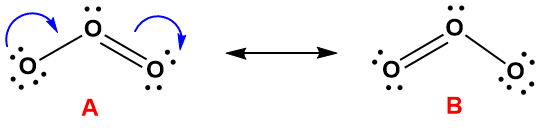 Figure 4-34. The interconversion of the two equivalent resonance structures for ozone. The blue arrows indicate the movement of electron pairs. For example, the conversion of structure A to structure B entails the creation of a double bond between central atom and the lower left using one of the latter's lone pairs, along with the shift of an electron pair from the original double bond to the oxygen on the right..The double-headed arrow in the figure is reserved for the depictions of resonance forms, it is never used to described reversible chemical reactions.
Figure 4-34. The interconversion of the two equivalent resonance structures for ozone. The blue arrows indicate the movement of electron pairs. For example, the conversion of structure A to structure B entails the creation of a double bond between central atom and the lower left using one of the latter's lone pairs, along with the shift of an electron pair from the original double bond to the oxygen on the right..The double-headed arrow in the figure is reserved for the depictions of resonance forms, it is never used to described reversible chemical reactions.
Structure A in Figure 4-34 is the same one we drew in Figure 4-33. But this structure can be modified by shifting electrons as indicated by the blue arrows. We will use a similar arrow notation when we discuss reaction mechanisms; the basic idea is that the tail of the arrow is positioned next to the original location of the electron pair, and the arrowhead indicates where they go. For example, to convert structure A to structure B, one of the electron pairs in the double bond is shifted to the oxygen on the right. If that was the only thing we did, the central oxygen would no longer have an octet, so at the same time we also form a new double bond using a lone pair on the left oxygen. In essence, electrons are flowing from left to right in the diagram. It is important to note, however that the only things moving between these forms are electrons - not any of the atoms. In other words, we are not simply flipping structure A 180° to get structure B, but it certainly looks like we did! Flipping the molecule would exchange the positions of the terminal atoms, and we are definitely not doing that. This is an important point that we will come back to.
If you consider structures A and B from Figure 4-34 individually, they both should have O-O-O bond angles of 120°, and they both should have one long bond and one short one. In that sense, they both correctly predict the bond angle but, on the other hand, they both fail to convey the equal lengths of the bonds. With that in mind, consider what the characteristics of the average between A and B might be. Start with the bond angle: the bond angle of an average between A and B would still be 120 because that value is shared by the two forms. Now consider bond length: if we look at the bond between the central oxygen and the one on the left, it has a bond order of 1 in structure A and 2 in structure B, making the average bond order is 11/2; if we did the same analysis on the bond on the right we would get a bond order of 11/2 again. Thus the average structure between A and B correctly predicts the geometry around the central oxygen, and also predicts oxygen-oxygen bond lengths that: a) are equivalent with each other, and b) have lengths of an intermediate value between the single and double oxygen-oxygen bonds. In other words, the average between A and B does agree with observation, despite the fact that the structures from which the average was obtained does not.
The above is an application of resonance theory. Think of it as being akin to a software "patch" that might be released to correct a flaw in an prematurely released computer program. Rather than making a program run with fewer glitches, this patch compensates for the overly restrictive requirement that bonding electrons must be localized between two adjacent atoms. In the example of ozone, you can view the electron pair that is involved in the double bond as being "smeared" out over all three oxygen atoms or, in other words, delocalized over the entire molecule. The individual structures, A and B, are called resonance forms or resonance structures. While each conveys the fact that all the atoms in the molecule obey the octet rule, they also result in a significantly distorted view of the structure because the flawed assumption that electrons must be localized between two atoms. So while structures A and B are both wrong in that they have two different type of bonds, taking the average of the two resonance structures corrects that problem. This averaged structure is called the resonance hybrid, and provides a more accurate depiction of the molecule (Figure 4-35). Note that if the double bond is spread out over the molecule, so too must be the lone pair that exchanges places with it when drawing the two resonance structures. There is no elegant way of showing the delocalized lone pairs, so electron dot diagrams of resonance hybrids often omit them.
 Figure 4-35. The resonance hybrid of the two distinct resonance structure shown in Figure 4-34. The bond order between each pair of adjacent oxygen atoms is 1.5, represented by the solid line and accompanying dashed line between the atoms. For clarity, lone pairs are often omitted in diagrams of resonance hybrids.
Figure 4-35. The resonance hybrid of the two distinct resonance structure shown in Figure 4-34. The bond order between each pair of adjacent oxygen atoms is 1.5, represented by the solid line and accompanying dashed line between the atoms. For clarity, lone pairs are often omitted in diagrams of resonance hybrids.
Let's pause here to emphasize some fundamental points and hopefully prevent some common misconceptions from taking root. First, it is important to recognize that the term resonance can be misleading: it is sometimes misinterpreted to mean that the various resonance structures are oscillating between each other. This is not true. Neither resonance form represents an actual structure, so there can be no back-and-forth interconversion of the two forms. In other words, structures that correspond to the individual resonance form don't exist! Structures that don't exist can't interconvert! They are figments of our imagination, imposed on us by an overly restrictive notion of what covalent bonds can be! But, while each of them is misleading, the each have enough underlying validity that the average between them - the resonance hybrid - provides a good model for the actual structure. We also stated above that the only difference between different resonance structures of the same species is the placement of electrons. This is critical: if the position of any atoms differ between electron dot diagrams, they are not resonance structures of each other. In other words, we can define resonance structures as: two or more electron dot diagrams that differ only with respect to the arbitrary placement of nonbonding electrons and/or multiple bonds. The diagrams A and B for ozone fit this definition. Finally, note the use of the double-headed arrow between the two resonance structures in Figure 4-34. This type of arrow is reserved for the sole purpose of depicting resonance forms; this prevents confusion between the depiction of resonance forms (neither of which depict actual species), and reversible reactions, where the reactants and products, as written, do represent actual species. As we shall soon see, the latter situation is depicted using two arrows pointing in in the forward and reverse directions.
At a Glance: Resonance
We pause here to recap some key ideas because resonance turns out to be a critical tool in analyzing organic structures, especially for unstable reaction intermediates. The key takeaway is this: electron dot diagrams that differ only by the arbitrary placement of lone pairs and/or multiple bonds are called resonance structures. When multiple resonance structures exist for a give molecule or polyatomic ion, a more accurate depiction of the bonding will be conveyed by the resonance hybrid. Multiple resonance structures of the same molecule or ion are usually depicted with a double-headed arrow ( \( \ce{ <-> } \) ) to indicate their relationship.
The example of ozone provides us with an opportunity to introduce a new concept, albeit one we have referred to previous to this point: formal charge. In the two resonance structures of ozone in Figure 4-34, you can see that the way the electrons are distributed among the three oxygen atoms is not the same. While they all have their octets satisfied, only one of the three oxygen follows its normal valence: the central oxygen has three bonds and one lone pair, while one of the terminal atoms has one bond and three lone pairs. This suggests that the overriding tendency for atoms of a given element is to achieve the octet and not necessarily to have a specific number of bonds. Octet trumps valence, in other words. The singly bonded oxygen atoms seem to "compensate" for the lack of their second bond by taking on an extra electron which, as you would suspect, gives them some excess negative charge. Conversely, the central oxygen compensates for its excessive number of bonds by having fewer lone pairs. This oxygen is electron deficient because it is sharing more of its electrons than oxygen normally would. Formal charge attempts to convey these differences in charge that arise from various modes of bonding. As we will see it is, as the term suggests, a formalism. It oversimplifies and therefore distorts the actual charges borne by individual atoms. But it adds an important dimension to how we think about structures and, despite its inaccuracy, can be extremely useful. It is defined in a fairly straightforward way and, once you see the pattern, is readily determined simply by inspection.
The formal charge of any atom simply indicates how many more (or fewer) valence electrons it has compared to a neutral atom of the same element. Let's illustrate using water. Three electron dot diagrams of water are shown below: one that omits the lone pairs, one that shows them explicitly and one that shows the shared electrons in the covalent bonds as dots. The latter is color coded, indicating which electrons "belong" to oxygen (blue) and which belong to hydrogen (red). In terms of "crediting" each atom with specific electrons, those in shared pairs are split equally, one electron to each atom. In the case of oxygen, it is assigned six (blue) valence electrons, the same number as neutral atom of oxygen. The formal charge on oxygen in water is therefore zero. Each hydrogen atom is assigned one electron, the same as a neutral atom of hydrogen, making its formal charge zero as well. As this example shows, and as you can prove to yourself, when an atom has its normal valence and satisfies the octet rule, it usually has a formal charge of zero.
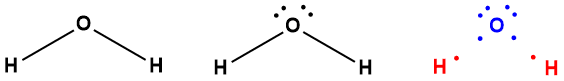 |
| Figure 4-36. Three electron dot structure of water. The structure on the right is helpful when determining the formal charges on the atoms. Because shared pairs are split 50/50, the oxygen atom has a total of six electrons (4 from the lone pairs, and 2 from the bonding pairs), giving it a formal charge of zero because neutral oxygen also has 6 valence electrons. The hydrogen atoms each have one electron, the same as a neutral atom of the element, making its formal charge zero as well. |
The approach we followed above can be summarized in the following formula, where FC stands for formal charge, and n is the number of valence electrons of a neutral atom of the element (which is the same the column number of the element):
\[ FC = n - [(number\ of\ unshared\ electrons) + 1/2 (number\ of\ shared\ electrons)] \]
The "1/2" term in the above equation arises from the fact that we are assigning half the number of shared electrons to each atom connected by covalent bonds. To illustrate how the above equation applies to hydrogen (in column 1A) and oxygen (in column 6A) in water:
\[ \begin{align*}
FC_{oxygen} &= 6 - [4 + 1/2 (4)] = 0 \\[5pt]
FC_{hydrogen} &= 1 - [0 + 1/2 (2)] = 0 \\[5pt]
\end{align*} \] While the above equation certainly works, it is often easier to get the formal charge by simple inspection. For a given atom, simply add the number of unpaired electrons and the number of covalent bonds, then subtract that sum from the column number of the element. For oxygen in water, there are 4 unshared electrons and 2 covalent bonds, giving us 6; subtract 6 from 6 and you get zero. For hydrogen, there are zero unshared electrons and one covalent bond; subtract 1 from 1 and you get the formal charge of zero.
 Using the same approach for the atoms in ozone, we can see how nonzero formal charge arise. To illustrate, we'll use one of resonance structure A for ozone from Figure 4-34 (shown at right). For clarity, we labeled the oxygen atoms a, b and c and we show how to determine the formal charges below for each one:
Using the same approach for the atoms in ozone, we can see how nonzero formal charge arise. To illustrate, we'll use one of resonance structure A for ozone from Figure 4-34 (shown at right). For clarity, we labeled the oxygen atoms a, b and c and we show how to determine the formal charges below for each one:
\[ \begin{align*}
FC_{O_{a}} &= 6 - [6 + 1/2 (2)] = -1 \\[5pt]
FC_{O_{b}} &= 6 - [2 + 1/2 (6)] = +1 \\[5pt]
FC_{O_{c}} &= 6 - [4 + 1/2 (4)] = 0 \\[5pt]
\end{align*} \]
The above indicates that oxygen atom c, the only one that achieves its octet and has its normal valence of two, has a zero formal charge. The other two have non-zero formal charges, one because it has an excess of electrons compared to neutral atoms of oxygen, and one is positive because it has fewer electrons than neutral atoms of oxygen. Specifically, oxygen atom a has a -1 formal charge because it has an "extra" lone pair of electrons to make up for not having its normal valence. Oxygen atom b, the central atom of ozone, has three covalent bonds and only one lone pair, making it electron deficient, a fact reflected by its +1 formal charge. Note that the some of the formal charges is zero, reflecting the fact that ozone is a neutral molecule overall. This is, in fact, an example of a general rule: the sum of the formal charges for all atoms in a molecule or polyatomic ion must be equal to its overall charge. We didn't point it out in the case of water, but it was true there as well: all three atoms had zero formal charges and the molecule has a whole is neutral. This can serve as a useful check on your work.
How literally should you take formal charges? It must be acknowledged that it is a crude way of assigning charge, and we have already introduced evidence that seems to question its accuracy. For example, we know water is polar in that the oxygen atom is slightly negative and the hydrogen is slightly positive. This is at odds with formal charges of zero on both elements. The reason for the discrepancy is easy enough to pinpoint: splitting the shared electrons exactly 50/50 between the atoms is an oversimplification. So why use formal charge? Don't mistake a lack of perfect accuracy with a lack of usefulness! Assigning formal charges is very easy and, despite the fact that it results in a distorted view of how electrons are shared, it can still provide valuable insights. To illustrate, let's go back to the resonance structures for ozone, but this time we'll write the formal charges associated with each of the atoms, as this will help us think about what the average charge distribution between them will be (Figure 4-37).
 |
| Figure 4-37. Left: The two resonance structures of ozone from Figure 4-34 with the non-zero formal charges shown next to the corresponding atoms. The charges shown for the resonance hybrid are the averages of the formal charges for each atom in the individual resonance structures. For example, the leftmost oxygen has a charge of -1 and 0 in structures A and B, respectively. The average of this is -1/2. The same result is obtained for the rightmost oxygen. The formal charge on the center oxygen is +1 in both resonance structures so the average in the hybrid is also +1. |
 Figure 4-38. Electrostatic potential map of ozone; the color coding indicates the relative charge at the surface of the molecule, with blue indicating partial positive charge and red indicating partial negative (the exact scale used for this depiction is shown to the left). The quantum mechanical calculations are consistent with the key qualitative insights suggested by the resonance hybrid, namely the positive charge on the central atom, the negative charge on the terminal atoms, as well as the symmetric charge distribution,..
Figure 4-38. Electrostatic potential map of ozone; the color coding indicates the relative charge at the surface of the molecule, with blue indicating partial positive charge and red indicating partial negative (the exact scale used for this depiction is shown to the left). The quantum mechanical calculations are consistent with the key qualitative insights suggested by the resonance hybrid, namely the positive charge on the central atom, the negative charge on the terminal atoms, as well as the symmetric charge distribution,..
Quantum mechanical calculations of the structure ozone are in qualitative agreement with our resonance hybrid. Figure 4-38 shows the electrostatic potential (esp) map of O3. Recall that we introduced esp maps back in Chapter 1 to illustrate polarity; the surface of the molecule is color-coded, with blue indicating more positive charge and red indicating a more negative charge. As you can see, the esp map reflects both the angular nature of the molecule as well as the relative charge imbalance the resonance hybrid predicted: the central atom is relatively positive and the terminal atoms are more negative, just as the formal charges in the hybrid indicated they would be. Moreover, the esp maps also indicates that the charge distribution is perfectly symmetrical: both terminal atoms appear to bear equal levels of negative charge. This means that the concept of the hybrid as being the average between the individual resonance structure is, if not correct, at least corroborated by much more sophisticated approaches to bonding.
The concept of resonance is particularly important for understanding the structures of many polyatomic ions, as well as unstable intermediates that form during the course of reactions pathways but are themselves not stable compounds. As such, we will be making extensive use of resonance throughout this book from this point on. Given the importance of the topic and its multiple applications, we want to show a few more examples of its application. We begin with carbonate, CO32-, perhaps the most widely encountered polyatomic ion in both biological and environmental contexts.
As we did with ozone, we will build the electron dot diagram of carbonate ion, CO32- using the electron dot approach (Figure 4-39) and then compare the resulting structure with what is actually observed. Much of the approach employed in the figure below is review but has the added twist of adding two "extra" electrons to achieve the -2 charge of the polyatomic ion. We'll also work with the assumption that the carbon is the central atom in the ion and all three of the oxygen atoms are terminal.

Figure 4-39. "Building" carbonate. a) start with electron dot structures of the neutral atoms; b) create the molecular skeleton by pairing unpaired electrons to make single bonds; c) add two electrons to achieve the necessary overall charge (CO32-); this completes the octets of two out of the three oxygen atoms; d) complete the octets of the central carbon and the electron-deficient oxygen by making a double bond.
To generate the electron dot diagram of carbonate, (a) we start with the neutral component atoms of carbonate (3 oxygen atoms and 1 carbon atom), and then (b) make the molecular skeleton by pairing unpaired electrons to form single bonds. The depiction labeled b) in the figure has one major problem that we need to address before going any further. Specifically, we started with 4 neutral atoms, meaning that we'll end up with a neutral CO3 molecule unless we add more electrons. We achieve the required -2 charge by adding two electrons, one each to two different oxygen atoms, thereby completing the octets of those atoms; the added negative charge borne by the two oxygen atoms (they each now have formal charges of -1) is explicitly signified by the negative signs next to those atoms in figure c). This leaves the remaining oxygen and the central carbon atom with seven electrons; making a double bond between those two will satisfy their octets, giving us a structure that satisfies the octets of all the atoms. Note that the formal charges of the carbon and double-bonded oxygen are both zero (they have their octets satisfied and their normal valence).
You should recognize that we made some arbitrary decisions in generating our electron dot diagram: this is another example of the sort of "red flag" we mentioned at the outset of this section. Specifically, the location of where we placed the extra electrons was completely random but, once that decision was made, it forced us to place the double bond where we did; if we had placed the electrons on different atoms, that would have affected the placement of the double bond. We should therefore be suspicious of the structural implications that we draw from the diagram. Specially, the electron dot diagram drawn predicts that carbonate should:
- have a trigonal planar geometry with O-C-O bond angles of 120°; this is because the steric number of the carbon is 3 and it has no lone pairs. And;
- have two carbon-oxygen bonds that are longer than the third; this is because the electron dot diagram as two single bonds and one double bond.
The observed bond angle of carbonate is in perfect accord with our prediction; it is indeed 120°. But, as we saw with ozone, the bond lengths suggested by the diagram are not observed. The carbon-oxygen bond lengths in carbonate are, in fact, identical, just as they were with ozone. Once again, to develop a better understanding of the structure of carbonate, we need to simultaneously examine all of the possibilities that exist when making the arbitrary decisions we described just above. There are three possible structures one could draw for carbonate, labeled A, B and C in Figure 4-40. If you consider each of these structures individually, they all should have O-C-O bond angles of 120°, and they all should have two longer carbon-oxygen bonds and one shorter one. What would the hybrid look like? If you can image the "average" of the three, there would be 11/3 bonds between the carbon and each oxygen. You can understand this as follows: pick any oxygen atom (e.g., the top one) and look at how it is bonded to the carbon in all three resonance structures. Regardless of which one you choose, it has single bonds to the carbon in two resonance forms and a double bond in the third. The average between 1, 1, and 2, is 11/3. Here's another way to look at it: regardless of which resonance form you look at, there are a total of 4 bonds between carbon and the 3 oxygen atoms, making the average bond order 11/3. Finally, and once again recalling what we saw with ozone, the formal charge on any specific atom of the hybrid can be found by averaging the formal charges of that atom in all the  resonance forms. Thus the formal charges on oxygen atoms is -2/3 in the hybrid, while that of carbon is zero. The sum of the formal charges is therefore -2, as it needs to be. A depiction of the resonance hybrid of carbonate, along with the quantum mechanical model of the ion are shown in Figure 4-41. Both indicate that the carbon-oxygen bonds have identical lengths and that the oxygen atoms are considerably more negative than the carbon and, moreover, that they have the same magnitude of negative charge. The resonance hybrid does indeed provide a more accurate depiction of the actual structure than any of the individual resonance forms.
resonance forms. Thus the formal charges on oxygen atoms is -2/3 in the hybrid, while that of carbon is zero. The sum of the formal charges is therefore -2, as it needs to be. A depiction of the resonance hybrid of carbonate, along with the quantum mechanical model of the ion are shown in Figure 4-41. Both indicate that the carbon-oxygen bonds have identical lengths and that the oxygen atoms are considerably more negative than the carbon and, moreover, that they have the same magnitude of negative charge. The resonance hybrid does indeed provide a more accurate depiction of the actual structure than any of the individual resonance forms.
Figure 4-40. The interconversion of the three equivalent resonance structures for carbonate. The blue arrows indicate the movement of electron pairs. For example, the conversion of structure A to structure B entails the creation of a double bond between the carbon and the lower right oxygen, using one of the latter's lone pairs, along with the movement of the electron pair in the original double bond to the topmost oxygen.
 |
| Figure 4-41. (left) The resonance hybrid of the carbonate ion, CO32-, showing the formal charges on each of the oxygen atoms; the bond order is 11/3. (center) A semitransparent esp map of carbonate, showing the position of the individual atoms within the electron cloud enveloping the ion. (right) The solid esp map accentuating the charge distributing at the surface of the molecule; blue is more positive (relatively speaking) and the yellow to red shades are more negative (scale shown). |
Problem 4-29. The nitrite ion has the formula NO2-. Using the approach employed in the analysis of carbonate, above, draw all the resonance structures for the nitrite ion and describe the resonance hybrid, specifically with respect to the nitrogen-oxygen bond order, the O-N-O bond angle, and the charges on the oxygen atoms. Assign formal charges to each atom in all the resonance forms and, from those, determine the charge distribution in the resonance hybrid.
The above examples of resonance have something in common with each other but which is not shared with many other molecules or polyatomic ions for which resonance is relevant. Specifically, in both cases, the various resonance structures for each species were equivalent with each other. What does equivalent mean in this context? It means that they had all the same feature in all the same quantities, and the only difference was their placement. In the case of ozone, both resonance structures have the following features: one single and one double oxygen-oxygen bond, a total of six lone pairs of electrons in the molecule, and formal charges of -1, 0, and +1 on the oxygen atoms. The arrangement of these features differ between the two forms, but the fact that they all have the same total number of bonds and lone pairs, as well as the same formal charges, makes them equivalent. The same is true for the carbonate resonance structures: they all have one carbon-oxygen double bond, two single bonds, a total of 8 lone pairs, and formal charges of 0 for the carbon and one oxygen, and -1 on the other two oxygen atoms. Here's why pointing out the equivalence of the resonance structures is important: equivalent resonance structures contribute equally to the resonance hybrid. Thus the hybrid for ozone was 50% structure A and 50% structure B from Figure 4-34. Similarly the hybrid for carbonate is composed of one-third of structures A, B and C in Figure 4-40.
 |
| Figure 4-42. Top: electron dot diagrams of CO2 and N2O, which are isoelectronic. Bottom: Three resonance structures of N2O. Structure B can be obtained from Structure A by shifting electrons to the right (blue arrows), while structure C can be obtained from structure A by shifting electrons to the left (red arrows). Formal charges are shown in gray. |
There are many occasions when resonance structures for a given species will not be equivalent. In those cases, the hybrid will be a weighted average of the various resonance structures, with the most stable resonance form having greater weight than less stable resonance forms. To illustrate, let's examine everyone's favorite nitrogen compound, nitrous oxide, N2O, which also goes by the names dinitrogen oxide and, more familiarly, "laughing gas". Nitrous oxide has 16 total valence electrons, the same as carbon dioxide. They are isoelectronic, in other words. As isoelectronic compounds, they may have a similar bonding structure. This is illustrate in the top of Figure 4-42. In it, we kept the positions of all the electrons exactly the same and only changed the identities of the atoms. Be careful here, though. Just because CO2 has two double bonds and is isoelectronic with N2O does not mean that they have entirely analogous structures. To see why, examine the formal charges of the atoms in CO2 and compare them to N2O. In CO2, all the atoms have formal charges of zero (they each obey the octet rule and they follow their normal valences). But there is no way to construct an electron dot diagram of N2O in which all of the atoms obey the octet rule and follow their normal valences. This results in multiple resonance forms with unequal energies – inequivalent resonance structures, in other words.
The resonance form of N2O that is most similar to the electron dot diagram of CO2, in which the electrons are evenly distributed across the molecule (two double bonds and two lone pairs on the terminal atoms) is labeled at structure A in Figure 4-42. It has a 0 formal charge on the oxygen, +1 on the central nitrogen, and -1 on the terminal nitrogen. Two other resonance forms exist. They can be generated by having the symmetrical electron distribution "flow" to the left or flow to the right, giving us another glimpse of the fluidity of electrons in molecules. The flow to the right is represented by the blue arrows on structure A in Figure 4-42; one of the lone pairs on the terminal nitrogen shifts to form a triple nitrogen-nitrogen bond, and an electron pair shifts from the double-bond on the right to the oxygen, yielding structure B. These shifts preserve the octets of all the atoms, but changes the formal charges (shown in gray). Starting with A again, a similar shift of electron to the left results in structure C, with formal charges of -2, +1, and +1 on the atoms. Note that these 3 resonance forms are not equivalent in that they have different types of bonds between the atoms as well as different formal charges.
How do we know which form, A, B or C, will be the major contributor to the hybrid? Think about it in terms of stability: the most stable resonance structure will be the most important contributor to the hybrid. What influences stability? A key insight comes from formal charges: nonzero formal charges indicate an imbalance in local charges. The very strong attraction between opposite charges means that the most stable arrangements of positively charged nuclei and negatively charged electrons is usually where there is neither an excess or deficiency of electrons around nuclei - in other words, the more atoms with non-zero formal charges, the less stable the resonance structure, and the greater the magnitude of non-zero formal charges, the less stable the resonance structure. Application of this concept means that structure C is the least stable of the the structures because all the atoms have non-zero formal charges, one of which is a very destabilizing -2. This means that structure C contributes the least amount to the hybrid.
How can we rank the relative stabilities of the remaining 2 structures? They each have one atom with a zero formal charge, and two others with formal charges of +1 and -1. The concept introduced above is therefore not helpful here. The question now becomes: "What is more stable, the nitrogen having the +1 formal charge and the oxygen having -1, or vice versa?", as that is the crucial distinction between structures A and B. To answer this question, we need to introduce a new concept, one of the most widely employed ideas in chemistry: electronegativity. Electronegativity is a measure of an element's tendency to attract bonding pairs of electrons close to their nuclei. Viewed from another perspective, it is ability of an element to stabilize negative charge. It is a ubiquitous concept in chemistry because it explains a host of phenomenon, including polarity. It is the greater electronegativity of oxygen compared to hydrogen in water that makes the O-H bond polar, with oxygen being partially negative and hydrogen being partially positive. The relevance to the problem at hand is clear: whichever element stabilizes negative charge more effectively is the one that should have the -1 formal charge.
The most common way of expressing electronegativities is numerically on a scale that goes roughly from 0.7 to 4, with the higher value indicating greater electronegativity. The electronegativities of elements is depicted on the color-coded Periodic Table below (Figure 4-43), with the deeper shading reflecting greater electronegativity. Notice that metallic elements tend to have low electronegativities, while nonmetallic elements have higher electronegativities. This is consistent with the observation that metals tend to lose electrons to nonmetals when they make binary compounds. For the question at hand, we need only focus on the fact that oxygen is more electronegative than nitrogen (3.44 vs 3.04), meaning that it is more capable of stabilizing a negative formal charge. Nitrogen, being less electronegative, is more capable of bearing a positive formal charge.
Figure 4-43. Electronegativity values of the main group elements and transition metals; values for helium, neon, argon, and radon have not been determined. Note the trends: in any given column, values tend to decrease as you go down, while across any given row, there is a general, if somewhat erratic, increasing trend. Thus fluorine has the highest electronegativity as it is in the upper right hand corner of the table, while francium has the lowest, being in the lower left hand corner.
What do we conclude? With the above considerations in mind, we expect that structure B is the most stable resonance structure and therefore will be the most important contributor to the resonance hybrid. In other words, we should expect that the observed structure of N2O should bear the closest resemblance to resonance form B. Specifically, the nitrogen-nitrogen bond will have characteristics more similar to triple bond than a double bond and the oxygen will bear a partial negative charge. The observed measurements on N2O do indeed reveal that it has an very short nitrogen-nitrogen bond, suggesting a nitrogen-nitrogen bond with a bond order of nearly 3. In addition, quantum mechanical calculations (Figure 4-44) indicate that the oxygen is the most negative atom in the molecule (as one would expect based on electronegativity) and the central nitrogen is by far the most positive, providing additional support for the importance of structure B.
It is worthwhile to note, however, that even though structure A is not the most important contributor to the hybrid, the actual structure does have some features indicated by this resonance form, namely the small but significant negative charge on the terminal nitrogen. This serves as an example that you shouldn't dismiss minor contributors to the resonance hybrid as being unimportant - just because they don't contribute as much to the hybrid as the most stable resonance form, doesn't mean that some of their characteristics won't be apparent in the actual structure.
How do the above observations relate to the concept of the hybrid being a weighted average of the individual resonance forms? Take the following numbers with a grain of salt: they are intended to illustrate the concept, not provide a rigorous quantitative treatment. With that caveat in mind, you can image the resonance hybrid of N2O as being, for example, composed of 75% structure B, 24% structure A, and 1% structure B. The resulting charges on the terminal N, central N, and O atoms with this weighting works out to be -0.26, +1, and -0.74, which is not too far off the quantum mechanical model illustrated in Figure 4-44. Regardless of the actual numbers used for the weighting, the key idea here is that resonance forms that are unequal will contribute differently to the hybrid, with the heavier weighting given to more stable resonance forms.
|
|
| Figure 4-44. Esp maps of N2O: (left) an opaque surface map showing the charge distribution at the surface of the molecule and (right) a semitransparent view that allows you to see the positions of the atoms. The oxygen is on the right and corresponds to the most negatively charged portion of the molecule; this indicates that resonance form B is the most important contributor to the hybrid. The central nitrogen is partially positive, while the terminal nitrogen is slightly negative, indicating that resonance form A also contributes to the hybrid, albeit to a lesser extent than B. |
Footnotes and References.
[25]. There is another way three neutral oxygen atoms could form a molecule in which all of the octets are satisfied. The two terminal oxygen atoms in the intermediate structure of Figure 4-33 both have one unpaired electron; if they were to form a bond with each other the result would be a closed, 3-membered ring. Such structures are known (cyclopropane is a known molecule, for example), but have considerable "ring strain" because the internal bond angles (60 for an equilateral triangle) are quite different from the ideal VSEPR angles. This results in much weaker bonds and greater instability. The structure of ozone, with its 117° O-O-O bond angle is not consistent with a closed ring but, in the absence of that experimental evidence, proposing such a structure is not unreasonable.


