4.8: Pulling It All Together - Molecular Topology
- Page ID
- 420704
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We've introduced a host of new topics since the start of this chapter: ionic and covalent bonds, valence electrons and the octet rule, the Periodic Table, molecular compounds, electron dot structures, molecular shapes and VSEPR, bond order and bond lengths, resonance, and formal charge. That's a lot to keep track of! We conclude this chapter with a topic that will, we hope, both reinforce many of the ideas already presented and will deepen your understanding of them by applying them to a new concept: molecular topology. As the term suggests, molecular topology refers to the physical landscape of molecules or, to put it less metaphorically, the arrangement of atoms in molecules with respect to their positions in the molecular skeleton. Our aim is to given you the tools to correctly predict what arrangement is the most stable for any given collection of atoms. In the previous section, we stated without explanation the molecular topology for two species: N2O and CO32-. We explain below why the particular order of atoms in these species is more stable than alternatives and, by doing so, will generate a list of guidelines that are broadly applicable to many other species. [26]
Let's start with N2O. Over the years we've noticed that many students initially assume the order of atoms in N2O is N-O-N, not N-N-O. It may have something to do with our instinctive preference for symmetry, or it could be due to students observe the fact that many compounds are arranged symmetrically, for example water and carbon dioxide and, as a result, and conclude that such symmetry is typical. But if you were to construct an electron dot diagram of NON, you soon run into some very unpalatable options. These are illustrated in three possible resonance forms of NON in Figure 4-45. Structure A is the result of simply making single bonds between the three atoms. There are actually some aspects of this structure that aren't bad: the formal charges on this structure are ideal - they are all zero - and the oxygen meets its normal valence of two. The nitrogen atoms, however, have not achieved their octets, which means they haven't decreased their potential energy as much as possible (recall that meeting the octet is a way of minimizing potential energy) and will therefore be expected to be highly reactive. Structure A, in other words, is not expected to be a stable species. Structure B is an improvement over A in that the octets of all the atoms are satisfied, but at what cost? The formal charges are very unfavorable: oxygen, the most electronegative element is bearing the +2 formal charge, while the nitrogen atoms, which are less electronegative, are each bearing -1 formal charges. This electron distribution is extremely unfavorable, negating the stabilizing effect of achieving the octets for each atom. Structure B, therefore, is not expected to be stable. Finally, in structure C, both nitrogen atoms achieve their normal valence of 3 and have zero formal charges, and the central oxygen also has a zero formal charge. But the oxygen has a greatly expanded octet, having six bonds! That is extremely energetically unstable. You may recall earlier that we stated that certain elements can expand their octets, but they are in the third, fourth and fifth rows of the Periodic Table. We will explain the quantum mechanical basis for oxygen's inability to expand its octet when we describe the electronic structure of atoms and molecules in greater detail; for now, however, all you need to know is that oxygen can not accommodate the twelve valence electrons it has in structure C.
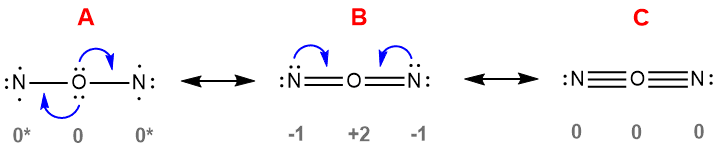 |
| Figure 4-45. Three resonance structures for the (non-existent) N-O-N structure of N2O. The only structure in which the octets are satisfied (B) for all three atoms has very unfavorable formal charges, while the structures that have optimal formal charges (A and C) do not allow octets for one or more atoms. |
None of the resonance forms in Figure 4-45 satisfactorily meet enough of the conditions for stability we've set forth prior to this point. They demonstrate that just having either ideal formal charges (all zero, as in A and C), or having the octet rule satisfied wherever possible (as in B), is insufficient to yield a reasonable resonance form. Both conditions need to be considered and, even it is not possible to meet them both perfectly, one or the other can not be grossly suboptimal. We saw a similar tension with the NNO structure in Figure 4-43, but in that case one of the resonance structures featured all of the atoms with complete octets and a reasonable array of formal charges (albeit some were non-zero). The avoidably poor charge distribution (as reflected by the poor formal charges) or inability to achieve the octet we see in NON, coupled with the fact that a reasonable alternative structure exists in NNO, explains the fact that the molecule NON has never been observed. The NON arrangement simply doesn't decrease the potential energy of those three atoms sufficiently.
As for carbonate, how can we rationalize the observation that the actual structure has a central carbon and three terminal oxygen atoms? To explain, let's compare the three electron dot diagrams in Figure 4-46, all of which have the formula CO32-. The diagram on the left is one of three equivalent resonance forms of carbonate while the other two represent alternative structures for the polyatomic ion – they have different molecular topologies in other words. You should notice that in all three diagrams, every atom obeys the octet rule, but structures corresponding to B and C have not been observed. The reason gets back to formal charge again; note that all of the formal charges sum to -2 as they must for polyatomic ions with an overall -2 charge. All the atoms of Structure B have non-zero formal charges, including both a -1 formal charge on the least electronegative atom (carbon) and a +1 formal charge on the most electronegative atom. That represents a highly destabilizing charge distribution. Structure C has fewer formal charges than B, but still has a -1 charge on the carbon and a +1 on the oxygen. Compare these to structure A which only has two atoms with non-zero formal charges, both -1 on the most electronegative atoms. In addition, as we've already seen, structure A is stabilized by two other equivalent resonance forms. Given this considerations, structure A is by far the most stable arrangement of this collection of particles (three oxygen atoms, one carbon atom and two additional electrons).
 |
| Figure 4-46. Three possible structures for a polyatomic ion with the formula CO32- that satisfy the octets of all four atoms. Only the structure on the left avoids a negative formal charge on the carbon (which has the lowest electronegativity). It also is the structure with the fewest non-zero formal charges, and the only structure that avoids placing lone pairs of electrons on adjacent atoms. |
The above two examples demonstrate that you could have predicted the structures of CO32- and N2O on the basis of the octet rule and formal charge considerations alone. Other The following delineates the general guidelines we've encountered that can guide you in determining the structures of other species. For a given formula for a molecule or polyatomic ion:
- atoms with higher valences tend to occupy central positions and atoms with lower valences tend to be terminal.
- whenever possible, the octet rule is followed by carbon, nitrogen, oxygen and fluorine. [27]
- topologies with the greatest number of atoms with a formal charge of zero tend to be more stable (this is the Pauling Electroneutrality Principle).
- when formal charges are unavoidable, the more positive formal charges should be on the least electronegative atoms and the negative formal charges should be on the most electronegative atoms.
- when +1 and -1 formal charges exist, topologies where they are on adjacent atoms are more stable than those when they are separated.
- topologies that afford multiple resonance forms, especially multiple equivalent resonance forms, are stabilized to greater extent than those that have fewer or no resonance forms.
The above points are loosely ranked in order of general importance, with the more dominant effects. We didn't mention valence in the discussions above, but atoms with higher valences tend to be more central because they are stabilized when making more bonds compared to atoms with lower valences. The correct structure of carbonate, for example, has carbon in the center where it can achieve its normal valence of 4, and oxygen is terminal, where it either meets its normal valence with a double bond, or achieves the octet by taking on a formal charge of -1. In the NON example, oxygen has a lower valence than nitrogen and therefore is not stable in the central position. The second and third items above are fairly self-explanatory at this point so we won't dwell on them.
The last bullet point above is particularly important and worth emphasizing because it arises quite often with polyatomic ions. The existence of multiple, equivalent resonance structures is highly stabilizing (the effect is often referred to as resonance stabilization) because it allows for the dispersal of formal charge over many atoms. Recall that the ideal formal charge on at atom, in terms of stability, is zero. Non-zero formal charges, such as those in carbonate, are reduced in the resonance hybrid meaning that the imbalance of charge is reduced. Other commonly encountered polyatomic ions also show resonance stabilization, including phosphate, sulfate, nitrate and carboxylate ions such as acetate.
The following series of steps can guide in predicting the most stable arrangement of atoms corresponding to a given formula for a molecule or polyatomic ion. It synthesizes the above points and incorporates ideas from the previous sections to you with the most likely structure, meaning the topology and important resonance forms, for a wide range of species. It is not universally applicable [28] and it is not foolproof [29], but it is enormously helpful for most molecules of biological significance. To determine the most stable arrangement of atoms in a molecule or polyatomic ion:
1. Build the molecular skeleton by placing atoms with higher valence (usually carbon or nitrogen) in more central positions and those of lower valence (usually hydrogen, or one of the halogens: fluorine, chlorine, bromine or iodine) in terminal positions. Oxygen can either be terminal or central, depending on formal charge considerations. The skeleton should be formed by making single bonds by pairing lone electrons from the neutral atoms. You may have to consider the formal charges of multiple possibilities to determine the most stable arrangement possible.
2. Add or remove electrons from the skeleton to achieve the stated charge of the species. Additional electrons should be placed on the more electronegative element that can accommodate it without exceeding its octet. If electrons must be removed, they should come from the least electronegative element that has nonbonding electrons.
3. Rearrange the electrons to achieve the octet for all terminal atoms (if possible); central atoms can expand their octet if they are in the third row of the periodic table or higher (e.g., P, S, Cl and higher atomic numbers).
4. If necessary, rearrange electrons to generate additional resonance structures. Using formal charges, determine the most important resonance forms using the Pauling Elelectroneutrality Principle
Let's see how the above guidelines, combined with the ideas from the previous sections, help us determine the optimal arrangement of atoms in a few species.
Problem 4-30. A polyatomic ion has a charge of -1 is composed of a of three atoms: one carbon, one nitrogen and one oxygen. What is the most stable arrangement of the atoms in this ion? What is the most important contributor to the resonance hybrid? What is the bond angle in this species?
Solution
Step 1: Assemble the molecular skeleton using neutral atoms. There are three possible atomic orders: CNO, CON, and NCO. Valence considerations favor the NCO ordering because that places the atom with the highest valence in the center. The other two possibilities places carbon, with the greater valence, in a terminal position and either nitrogen or oxygen, with lower valences, in the central spot.

Step 2: Add or subtract electrons to achieve the required charge. In this case place one additional electron on the oxygen atom because it is the most electronegative element.
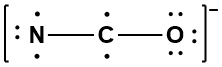
Step 3: Arrange the electrons to achieve the octets of all the terminal atoms and the central atoms. If necessary, consider different resonance forms to determine the most important contributor to the resonance hybrid. While two additional resonance forms exist in which the octets of all three atoms are satisfied, the resonance form shown has the best formal charges possible and will be the major contributor to the resonance hybrid.
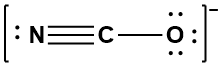
This is the isocyanate ion; the NCO bond angle is 180° and therefore has a linear molecular geometry.
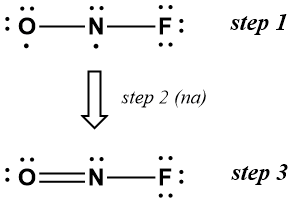
Problem 4-31. A neutral molecule consists of one atom each of nitrogen, oxygen, and fluorine. What is the most likely arrangement of these atoms? Describe the molecular structure.
Solution
The solution is shown below without further explanation (except for the cryptic note that Step 2 is not applicable in this case because the molecule is neutral). The molecular should have an angular geometry with an O-N-F bond angle of about 120°.
Problem 4-32. The compound dinitrogen pentoxide (N2O5) is known. Based on molecular topology considerations, predict what the most likely structure of this compound is. Estimate all of the O-N-O bond angles and any other bond angles that may exist.
The ideas presented above are not only useful to predict the structures of stable compounds, but of highly reactive species as well. For example, many chemical reactions take place via a series of simple steps that can involve the generation of high energy transient species (called intermediates) that are subsequently consumed in later steps. The structure of such species can be surmised using the same principles used above. Figure 4-47 illustrates one such simple step, the reversible reaction of a carbonyl functional group with an acid. Specifically, H2SO4 (sulfuric acid) donates hydrogen ion (H+) to acetone (which is an example of a ketone, a functional group we haven't introduced yet), resulting in HSO4- (called hydrogen sulfate, consisting of the original molecule minus the H+) and a product with the formula (CH3)2COH+. We'll discuss acid/base chemistry soon but, for now, focus on how the H+ ion might form a bond to the carbonyl group of acetone. Two possibilities exist: the hydrogen atom will be connected to either the carbon or the oxygen, but which one? Both possibilities are shown in the figure. You can view these structures as resulting from an "attack" of the H+ on the double bond. We stated back in Chapter 1 that double bonds have electrons that are more loosely held than those in single bonds and are therefore "vulnerable" to attack by species attracted to negative charges. The H+ ion, which has no electrons of its own, is such a species and can redirect two of the electrons in the double bond, converting them to a new single bond connecting the hydrogen atom to the molecule. In structure A, the H+ bonds to the oxygen while in B, it bonds to the carbon. Note that it can't attach to the methyl groups because they have no loosely held pairs of electrons (like lone pairs or double bonds) so are unreactive toward the acid.
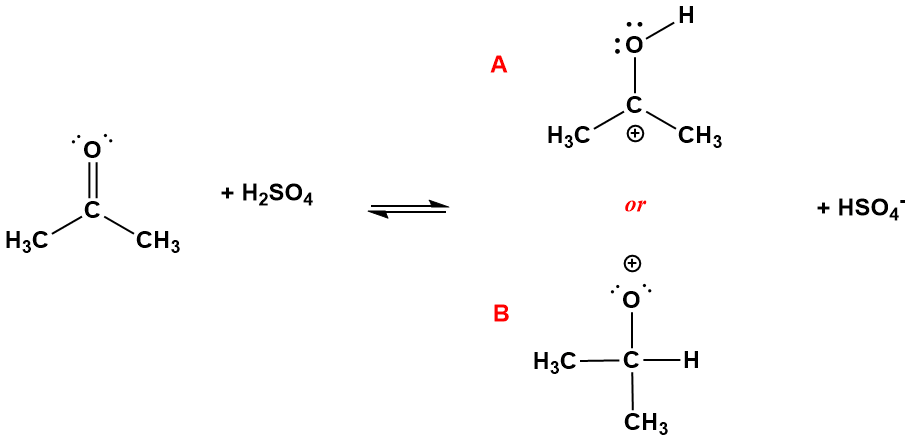
Figure 4-47. The reaction of acetone, (CH3)2CO, with sulfuric acid (H2SO4) involves the transfer of a hydrogen ion (H+) from the latter to the former. Two possible products can be formed, labeled A and B. Neither structure has complete octets for the all atoms: in A oxygen has a complete octet but carbon does not, while in B the reverse is true. A, however has a resonance form that satisfies the octets for both. Thus A is formed but B, which is not as stable, is not.
How can we predict which species A or B, will be formed? This is essentially a molecular topology problem: we have two different arrangements of atoms, one of which is more stable (and therefore more likely) to be formed than the other. Neither of which have complete octets and, as a result, we would not expect these to be stable species. But of the two, which is preferable? Structure A has the formal charge on the carbon, the less electronegative atom, while B has it on the more electronegative oxygen. This would favor A. However, an even more important consideration is this: structure A is resonance stabilized, while structure B is not (Figure 4-48). Specifically, shifting one of the lone pairs on oxygen to make a double bond with carbon gives a resonance form that satisfies the octets for both carbon and oxygen, albeit at the expense of shifting the formal charge to oxygen. Structure B has no viable resonance form to disperse the positive charge (you should prove this to yourself), so the positive charge would be borne solely by the most electronegative atom. We conclude, therefore, that the hydrogen will bond to the oxygen atom in this reaction because the alternative results in a much less stable product.
With respect to the resonance hybrid for structure A, which resonance form is the major contributor to it? The complete octets of the form on the right more than compensate for the poor formal charge making it the major contributor. But the minor contributor is important as well and it indicates that there will be significant positive charge on the oxygen, a fact that is highly relevant to the follow-up steps this species undergoes.

Figure 4-48. Two resonance forms for species A from Figure 4-48. Note that the structure on the right has complete octets for both carbon and oxygen which more than compensates for the positive formal charge on oxygen. making it the major contributor to the hybrid.
Footnotes and References
[26] The discussion in this section is inspired by a section in Chemical Structure and Bonding by Harry B. Gray and Roger L. DeKock (1980, Benjamin Cummings) entitled "The Use of Lewis Structures to Predict Molecular Topology".
[27] While some atoms can expand their octets, they usually only do so when they occupy the central position and when bound to very electronegative elements such as oxygen and fluorine. The phosphate, sulfate, and perchlorate ions, PO43-, SO42- and ClO4-, respectively, as well as neutral compounds like and PF5 and SF6, are all good examples of this. That said, for molecules of biological interest (other than phosphate!), focusing on elements that cannot expand their octets is quite reasonable.

