1.5: Applications of the Ideal Gas Law
- Page ID
- 164731
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To relate the amount of gas consumed or released in a chemical reaction to the stoichiometry of the reaction.
- To understand how the ideal gas equation can be used to calculate the density and molar mass of a gas.
With the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to calculate the stoichiometry of reactions involving gases, if the pressure and temperature are known. This is important for several reasons. Many reactions that are carried out in the laboratory involve the formation or reaction of a gas, so chemists must be able to quantitatively treat gaseous products and reactants as readily as they quantitatively treat solids or solutions. Furthermore, many, if not most, industrially important reactions are carried out in the gas phase for practical reasons. Gases mix readily, are easily heated or cooled, and can be transferred from one place to another in a manufacturing facility via simple pumps and plumbing.
Determining Gas Volumes in Chemical Reactions
Chemical stoichiometry describes the quantitative relationships between reactants and products in chemical reactions. We have previously measured quantities of reactants and products using masses for solids and volumes in conjunction with the molarity for solutions; now we can also use gas volumes to indicate quantities. If we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate how many moles of the gas are present. If we know how many moles of a gas are involved, we can calculate the volume of a gas at any temperature and pressure.
Example \(\PageIndex{1}\)
What volume of carbon dioxide gas is produced at STP by the decomposition of 0.150 g \(\ce{CaCO_3}\) via the equation:
\[ \ce{CaCO3(s) \rightarrow CaO(s) + CO2(g)} \]
SOLUTION
Begin by converting the mass of calcium carbonate to moles.
\[ \dfrac{0.150\;g}{100.1\;g/mol} = 0.00150\; mol \]
The stoichiometry of the reaction dictates that the number of moles \(\ce{CaCO_3}\) decomposed equals the number of moles \(\ce{CO2}\) produced. Use the ideal-gas equation to convert moles of \(\ce{CO2}\) to a volume.
\[ \begin{align*} V &= \dfrac{nRT}{R} \\[4pt] &= \dfrac{(0.00150\;mol)\left( 0.08206\; \frac{L \cdot atm}{mol \cdot K} \right) ( 273.15\;K)}{1\;atm} \\[4pt] &= 0.0336\;L \; or \; 33.6\;mL \end{align*}\]
Example \(\PageIndex{2}\): Sulfuric Acid
Sulfuric acid, the industrial chemical produced in greatest quantity (almost 45 million tons per year in the United States alone), is prepared by the combustion of sulfur in air to give SO2, followed by the reaction of SO2 with O2 in the presence of a catalyst to give SO3, which reacts with water to give H2SO4. The overall chemical equation is as follows:
\[\ce {2S(s) + 3O2(g) + 2H2O(l) \rightarrow 2H2SO4(aq)} \]
What volume of O2 (in liters) at 22°C and 745 mmHg pressure is required to produce 1.00 ton (907.18 kg) of H2SO4?
Given: reaction, temperature, pressure, and mass of one product
Asked for: volume of gaseous reactant
Strategy:
A Calculate the number of moles of H2SO4 in 1.00 ton. From the stoichiometric coefficients in the balanced chemical equation, calculate the number of moles of O2 required.
B Use the ideal gas law to determine the volume of O2 required under the given conditions. Be sure that all quantities are expressed in the appropriate units.
Solution:
mass of H2SO4 → moles H2SO4 → moles O2 → liters O2A We begin by calculating the number of moles of H2SO4 in 1.00 ton:
\[\rm\dfrac{907.18\times10^3\;g\;H_2SO_4}{(2\times1.008+32.06+4\times16.00)\;g/mol}=9250\;mol\;H_2SO_4\]
We next calculate the number of moles of O2 required:
\[\rm9250\;mol\;H_2SO_4\times\dfrac{3mol\; O_2}{2mol\;H_2SO_4}=1.389\times10^4\;mol\;O_2\]
B After converting all quantities to the appropriate units, we can use the ideal gas law to calculate the volume of O2:
\[V=\dfrac{nRT}{P}=\rm\dfrac{1.389\times10^4\;mol\times0.08206\dfrac{L\cdot atm}{mol\cdot K}\times(273+22)\;K}{745\;mmHg\times\dfrac{1\;atm}{760\;mmHg}}=3.43\times10^5\;L\]
The answer means that more than 300,000 L of oxygen gas are needed to produce 1 ton of sulfuric acid. These numbers may give you some appreciation for the magnitude of the engineering and plumbing problems faced in industrial chemistry.
Exercise \(\PageIndex{2}\)
Charles used a balloon containing approximately 31,150 L of H2 for his initial flight in 1783. The hydrogen gas was produced by the reaction of metallic iron with dilute hydrochloric acid according to the following balanced chemical equation:
\[\ce{ Fe(s) + 2 HCl(aq) \rightarrow H2(g) + FeCl2(aq)} \]
How much iron (in kilograms) was needed to produce this volume of H2 if the temperature was 30°C and the atmospheric pressure was 745 mmHg?
- Answer
-
68.6 kg of Fe (approximately 150 lb)
Gas Densities and Molar Mass
The ideal-gas equation can be manipulated to solve a variety of different types of problems. For example, the density, \(\rho\), of a gas, depends on the number of gas molecules in a constant volume. To determine this value, we rearrange the ideal gas equation to
\[\dfrac{n}{V}=\dfrac{P}{RT}\label{10.5.1}\]
Density of a gas is generally expressed in g/L (mass over volume). Multiplication of the left and right sides of Equation \ref{10.5.1} by the molar mass in g/mol (\(M\)) of the gas gives
\[\rho= \dfrac{g}{L}=\dfrac{PM}{RT} \label{10.5.2}\]
This allows us to determine the density of a gas when we know the molar mass, or vice versa.
Example \(\PageIndex{3}\)
What is the density of nitrogen gas (\(\ce{N_2}\)) at 248.0 Torr and 18º C?
SOLUTION
Step 1: Write down your given information
- P = 248.0 Torr
- V = ?
- n = ?
- R = 0.0820574 L•atm•mol-1 K-1
- T = 18º C
Step 2: Convert as necessary.
\[(248 \; \rm{Torr}) \times \dfrac{1 \; \rm{atm}}{760 \; \rm{Torr}} = 0.3263 \; \rm{atm} \]
\[18\,^oC + 273 = 291 K \]
Step 3: This one is tricky. We need to manipulate the Ideal Gas Equation to incorporate density into the equation.
Write down all known equations:
\[PV = nRT \]
\[\rho=\dfrac{m}{V} \]
where \(\rho\) is density, \(m\) is mass, and \(V\) is volume.
\[m=M \times n onumber\]
where \(M\) is molar mass and \(n\) is the numbe r of moles.
Now take the definition of density (Equation \ref{10.5.1})
\[\rho=\dfrac{m}{V} \]
Keeping in mind \(m=M \times n\)...replace \((M \times n)\) for \(mass\) within the density formula.
\[\rho=\dfrac{M \times n}{V} \]
\[\dfrac{\rho}{M} = \dfrac{n}{V} \]
Now manipulate the Ideal Gas Equation
\(PV = nRT \)
\[\dfrac{n}{V} = \dfrac{P}{RT} \]
\((n/V)\) is in both equations.
\[ \begin{align*} \dfrac{n}{V} &= \dfrac{\rho}{M} \\[4pt] &= \dfrac{P}{RT} \end{align*}\]
Now combine them please.
\[\dfrac{\rho}{M} = \dfrac{P}{RT} \]
Isolate density.
\[\rho = \dfrac{PM}{RT} \]
Step 4: Now plug in the information you have.
\[ \begin{align*} \rho &= \dfrac{PM}{RT} \\[4pt] &= \dfrac{(0.3263\; \rm{atm})(2*14.01 \; \rm{g/mol})}{(0.08206\, L\, atm/K mol)(291 \; \rm{K})} \\[4pt] &= 0.3828 \; g/L \end{align*}\]
The density of a gas INCREASES with increasing pressure and DECREASES with increasing temperature
An example of varying density for a useful purpose is the hot air balloon, which consists of a bag (called the envelope) that is capable of containing heated air. As the air in the envelope is heated, it becomes less dense than the surrounding cooler air (Equation \(\ref{10.5.2}\)), which is has enough lifting power (due to buoyancy) to cause the balloon to float and rise into the air. Constant heating of the air is required to keep the balloon aloft. As the air in the balloon cools, it contracts, allowing outside cool air to enter, and the density increases. When this is carefully controlled by the pilot, the balloon can land as gently as it rose.

Another useful application of the ideal gas law involves the determination of molar mass. By definition, the molar mass of a substance is the ratio of its mass in grams, m, to its amount in moles, n:
The ideal gas equation can be rearranged to isolate n:
and then combined with the molar mass equation to yield:
This equation can be used to derive the molar mass of a gas from measurements of its pressure, volume, temperature, and mass.
Example \(\PageIndex{4}\): Determining the Molar Mass of a Volatile Liquid
The approximate molar mass of a volatile liquid can be determined by:
- Heating a sample of the liquid in a flask with a tiny hole at the top, which converts the liquid into gas that may escape through the hole
- Removing the flask from heat at the instant when the last bit of liquid becomes gas, at which time the flask will be filled with only gaseous sample at ambient pressure
- Sealing the flask and permitting the gaseous sample to condense to liquid, and then weighing the flask to determine the sample’s mass (Figure \(\PageIndex{1}\))
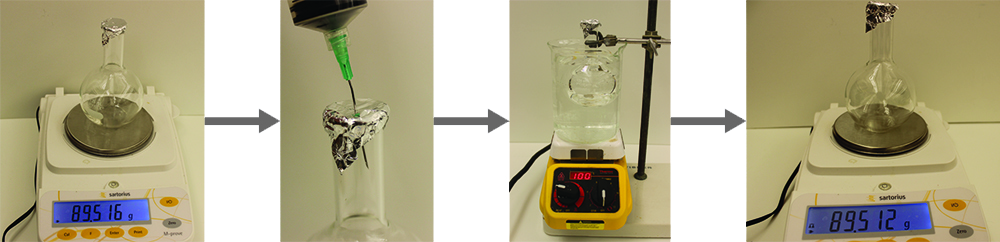
Using this procedure, a sample of chloroform gas weighing 0.494 g is collected in a flask with a volume of 129 cm3 at 99.6 °C when the atmospheric pressure is 742.1 mm Hg. What is the approximate molar mass of chloroform?
Solution
Since
\[M=\dfrac{m}{n} \]
and
\[n=\dfrac{PV}{RT} \]
substituting and rearranging gives
\[M=\dfrac{mRT }{PV}\]
then
\[M=\dfrac{mRT}{PV}=\mathrm{\dfrac{(0.494\: g)×0.08206\: L⋅atm/mol\: K×372.8\: K}{0.976\: atm×0.129\: L}=120\:g/mol} \]
Exercise \(\PageIndex{4}\)
A sample of phosphorus that weighs 3.243 × 10−2 g exerts a pressure of 31.89 kPa in a 56.0-mL bulb at 550 °C. What are the molar mass and molecular formula of phosphorus vapor?
- Answer
-
124 g/mol P4
Summary
The relationship between the amounts of products and reactants in a chemical reaction can be expressed in units of moles or masses of pure substances, of volumes of solutions, or of volumes of gaseous substances. The ideal gas law can be used to calculate the volume of gaseous products or reactants as needed.
The ideal gas law can also be rearranged to determine the density and molar mass of a gas when its mass is known under a given set of conditions.
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Anna Christianson, Bellarmine University

