21.6 Chemistry of Esters
- Page ID
- 90997
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester.
- write an equation to describe the hydrolysis of an ester under acidic or basic conditions.
- identify the products formed from the hydrolysis of an given ester.
- identify the reagents that can be used to bring about ester hydrolysis.
- identify the structure of an unknown ester, given the products of its hydrolysis.
- write the mechanism of alkaline ester hydrolysis.
- write the mechanism of acidic ester hydrolysis.
- write an equation to describe the reduction of an ester with lithium aluminum hydride.
- identify the product formed from the reduction of a given ester (or lactone) with lithium aluminum hydride.
- identify the ester, the reagents, or both, that should be used to prepare a given primary alcohol.
- write a detailed mechanism for the reduction of an ester by lithium aluminum hydride.
- identify diisobutylaluminum hydride as a reagent for reducing an ester to an aldehyde, and write an equation for such a reaction.
- write an equation to describe the reaction of an ester with a Grignard reagent.
- identify the product formed from the reaction of a given ester with a given Grignard reagent.
- identify the ester, the Grignard reagent, or both, needed to prepare a given tertiary alcohol.
- write a detailed mechanism for the reaction of an ester with a Grignard reagent.
Make certain that you can define, and use in context, the key terms below.
- lactone
- saponification
Many esters have characteristic aromas and flavours. Some examples are listed below.
Basic structure:
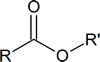
| IUPAC name | R | R′ | Aroma |
|---|---|---|---|
| octyl ethanoate | CH3 | CH3(CH2)6CH2 | orange |
| propyl ethanoate | CH3 | CH3CH2 CH2 | pear |
| 2‑methylpropyl propanoate | CH3CH2 | (CH3)2CHCH2 | rum |
| methyl butanoate | CH3CH2CH2 | CH3 | apple |
| ethyl butanoate | CH3CH2CH2 | CH3CH2 | pineapple |
A “lactone” is a cyclic ester and has the general structure
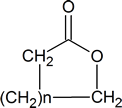
By recognizing that the steps in the acidic hydrolysis of an ester are exactly the same as those in a Fischer esterification (but in the reverse order!), you can again minimize the amount of memorization that you must undertake. The details of both mechanisms can be deduced from the knowledge that both reactions are acid‑catalyzed nucleophilic acyl substitutions.
Introduction
Esters are readily synthesized and naturally abundant contributing to the flavors and aromas in many fruits and flowers.
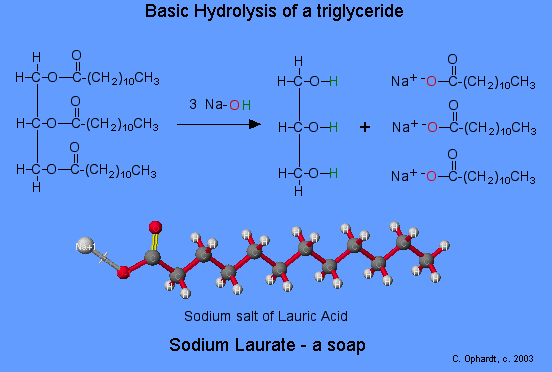
They also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Soap is produced by a saponification (basic hydrolysis) reaction of a fat or oil.
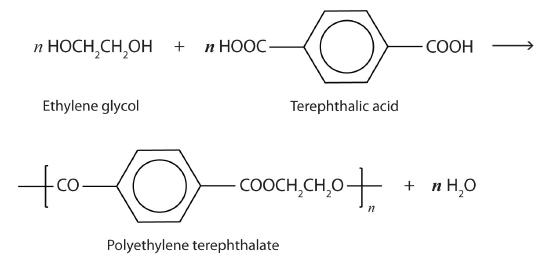
Esters are also present in a number of important biological molecules and have several commercial and synthetic application. For example, polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda pop and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes. Synthetic arteries can be made from PET, polytetrafluoroethylene, and other polymers.
The most important polyester, polyethylene terephthalate (PET), is made from terephthalic acid and ethylene glycol monomers:
Preparation of Esters
Carboxylic acids can react with alcohols to form esters
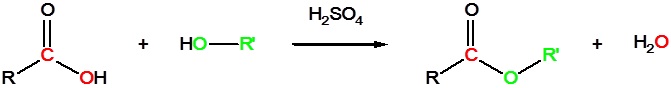
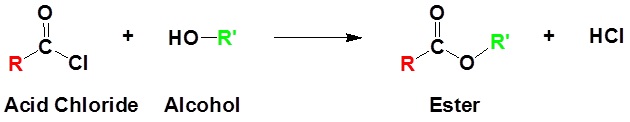
Acid chlorides react with alcohols to form esters
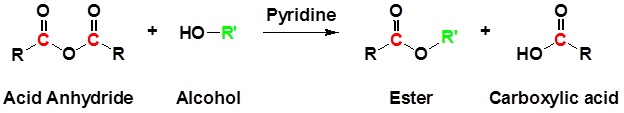
Acid Anhydrides react with alcohols to form esters
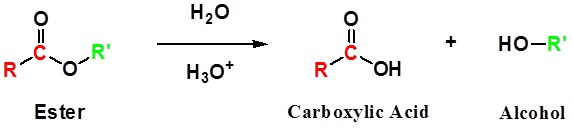
Conversion of Ester into Carboxylic acids: Hydrolysis
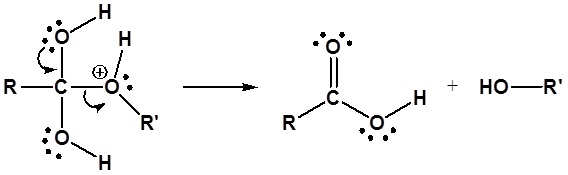
Esters can be cleaved back into a carboxylic acid and an alcohol by reaction with water and a catalytic amount of acid.
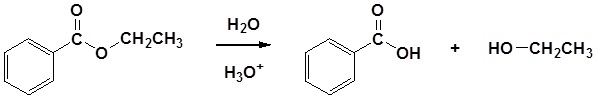
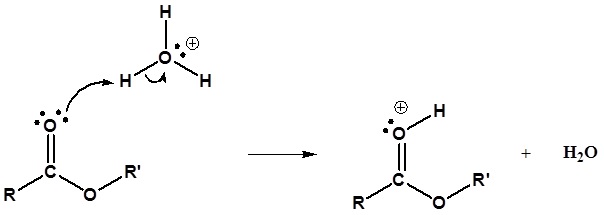
Mechanism
1) Protonation of the Carbonyl
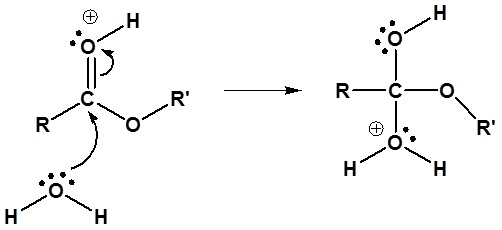
2) Nucleophilic attack by water
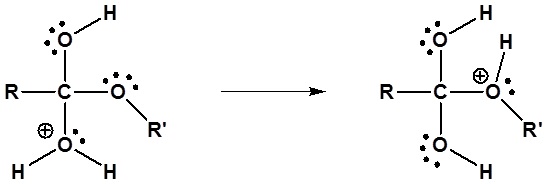
3) Proton transfer
4) Leaving group removal
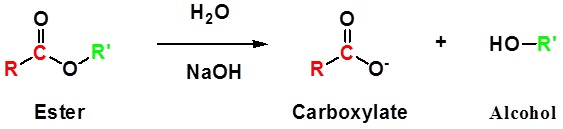
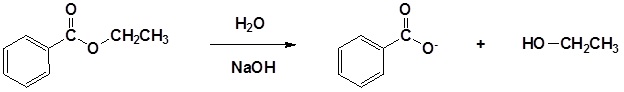
Esters can be cleaved back into a carboxylic acid and an alcohol by reaction with water and a base. The reaction is called a saponification from the Latin sapo which means soap. The name comes from the fact that soap used to me made by the ester hydrolysis of fats. Due to the basic conditions a carboxylate ion is made rather than a carboxylic acid.
Mechanism
1) Nucleophilic attack by hydroxide
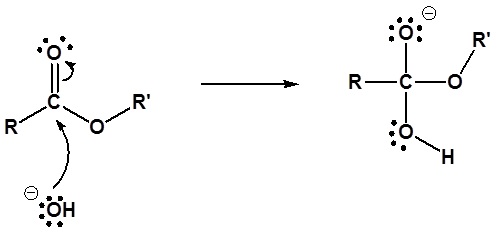
2) Leaving group removal
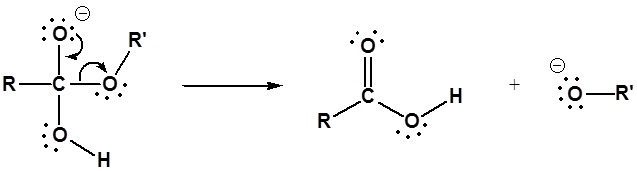
3) Deprotonation
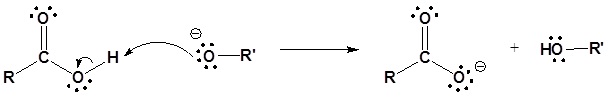
Conversion of Esters into Amides: Aminolysis
Esters reaction with ammonia and alkyl amines to yield amides.
Conversion of Ester into Alcohols: Reduction
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride (\(NaBH_4\)) is not a strong enough reducing agent to perform this reaction.
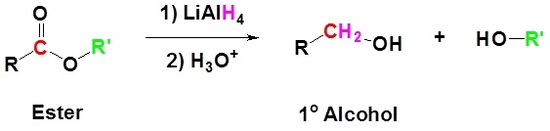
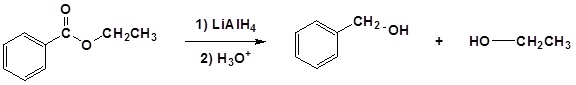
Mechanism
1) Nucleophilic attack by the hydride
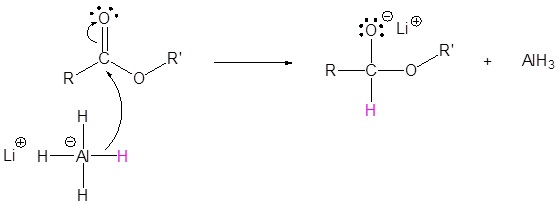
2) Leaving group removal
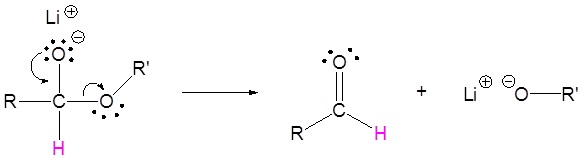
3) Nucleopilic attack by the hydride anion
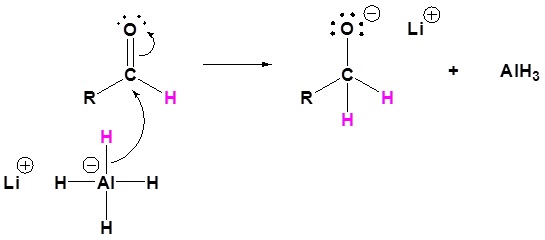
4) The alkoxide is protonated
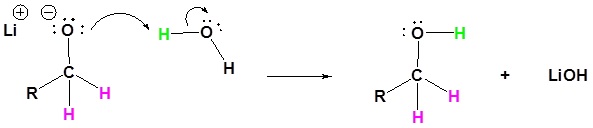
Esters can be converted to aldehydes using diisobutylaluminum hydride (DIBAH)
The reaction is usually carried out at -78 oC to prevent reaction with the aldehyde product.
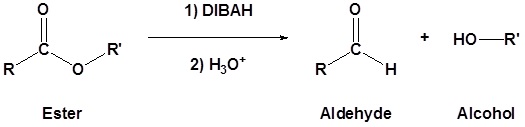
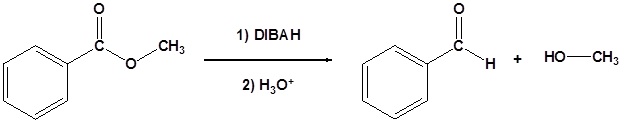
Addition of Grignard reagents convert esters to 3o alcohols.
In effect the Grignard reagent adds twice.
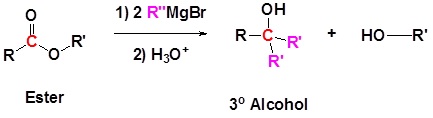
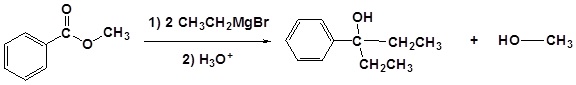
Mechanism
1) Nucleophilic attack
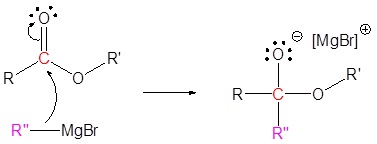
2) Leaving group removal
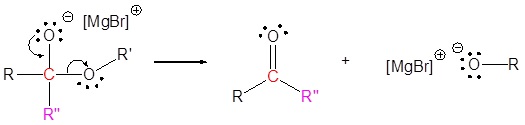
3) Nucleophilic attack
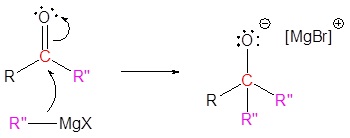
4) Protonation
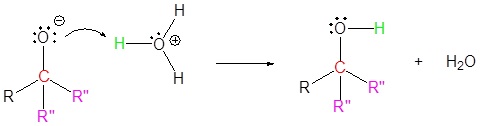
Exercises
Why is the alkaline hydrolysis of an ester not a reversible process? Why doesn't the reaction with a hydroxide ion and a carboxylic acid
produce an ester?
- Answer
-
The reaction between a carboxylic acid and a hydroxide ion is an acid base reaction, which produces water and a carboxylate anion.
Draw the product of the reaction between the following molecule and LiAlH, and the product of the reaction between the following
molecule and DIBAL.
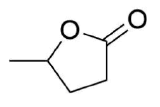
- Answer
-
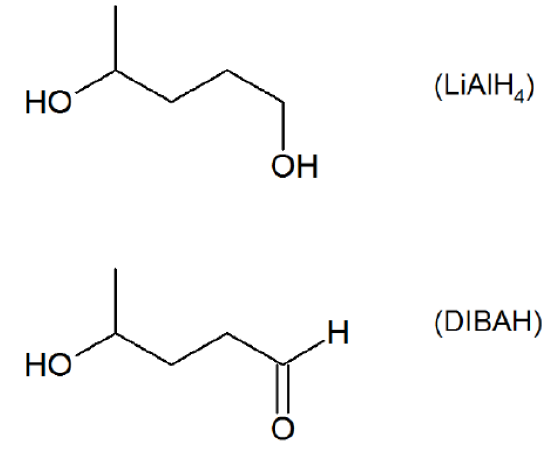
How might you Prepare the following molecules from esters and Grignards?
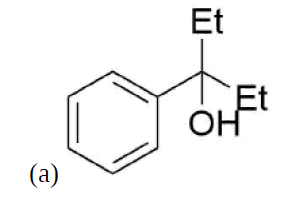
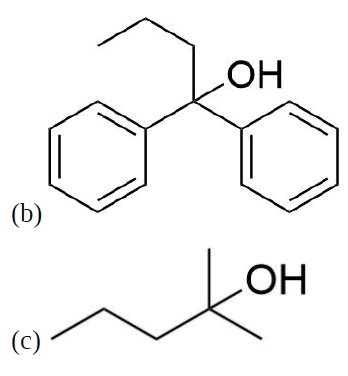
- Answer
-
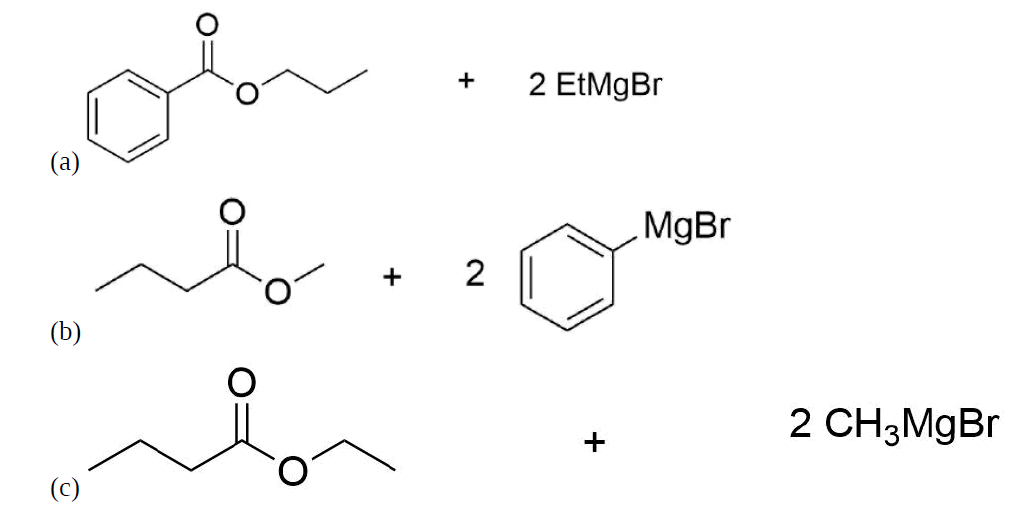
.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook