21.5 Chemistry of Acid Anhydrides
- Page ID
- 90996
Objectives
After completing this section, you should be able to
- write an equation to illustrate the preparation of an acid anhydride from an acid halide and the sodium salt of a carboxylic acid.
- identify the product formed from the reaction of a given acid halide with the sodium salt of a given carboxylic acid.
- identify the acid halide, carboxylate salt, or both, required to prepare a given acid anhydride.
- write an equation to describe the reaction of an acid anhydride with each of the following: water, alcohol, ammonia, a primary or secondary amine, lithium aluminum hydride.
- identify the product formed when a given acid anhydride is reacted with any of the reagents listed in Objective 4, above.
- write a detailed mechanism for the reaction of an acid anhydride with any of the reagents listed in Objective 4, above.
- identify the acid anhydride, the nucleophilic reagent, or both, needed to prepare a specified carboxylic acid, ester, amide, or primary alcohol.
The reactions described in this section are, in principle, identical to those discussed in Section 21.4. Once you have understood the mechanism of nucleophilic acyl substitution, these reactions should not present you with any great difficulty, and memorization can be kept to a minimum.
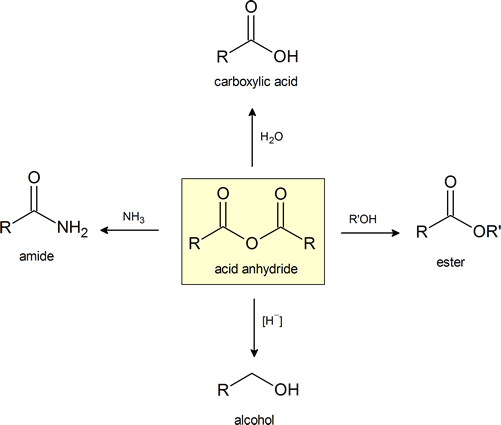
This figure provides a convenient general summary of a few of the reactions described in Section 21.5. Note that from a synthetic perspective the ester‑ and amide‑forming reactions are the most common, so they are the focus of this section.
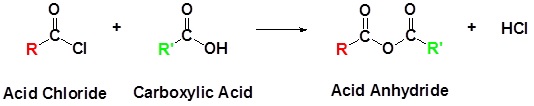
Preparation of Acid Anhydrides
Acid Chlorides react with carboxylic acids to form anhydrides
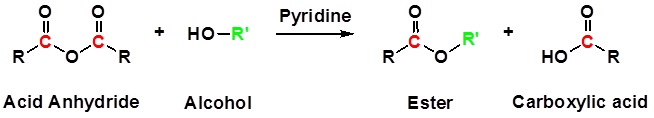
Acid Anhydrides react with alcohols to form esters
Reactions of anhydrides use Pyridine as a solvent

Mechanism
1) Nucleophilic Attack by the Alcohol
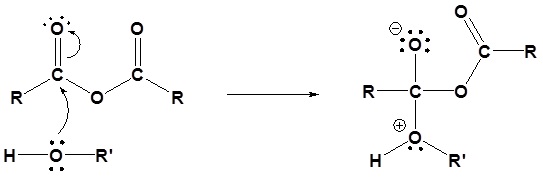
2) Deprotonation by pyridine
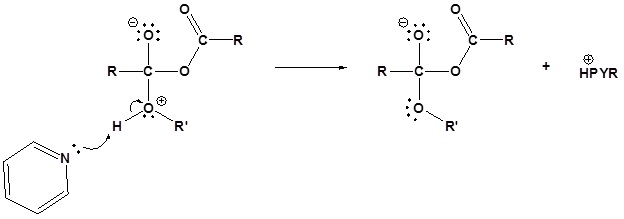
3) Leaving group removal
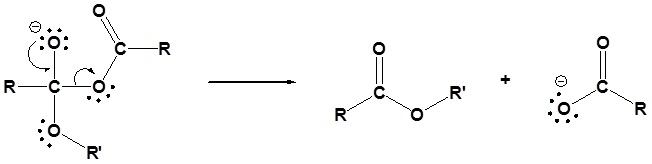
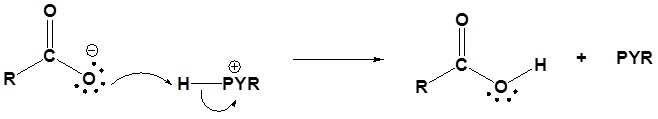
4) Protonation of the carboxylate
Acid Anhydrides react with amines to form amides
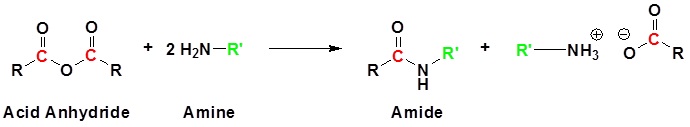
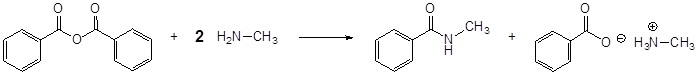
Mechanism
1) Nucleophilic Attack by the Amine
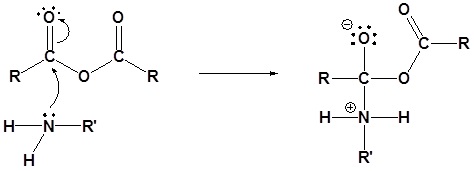
2) Deprotonation by the amine
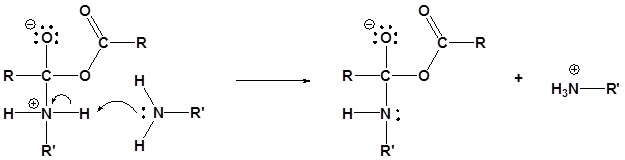
3) Leaving group removal
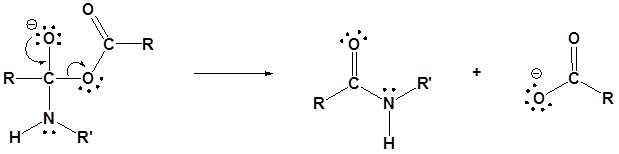
Exercises
Draw out the mechanism for the following reaction.
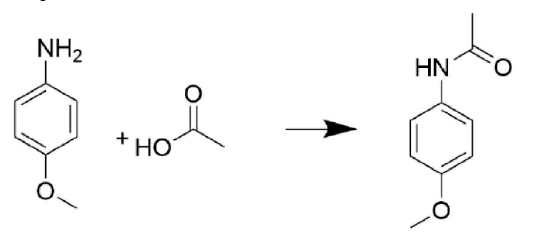
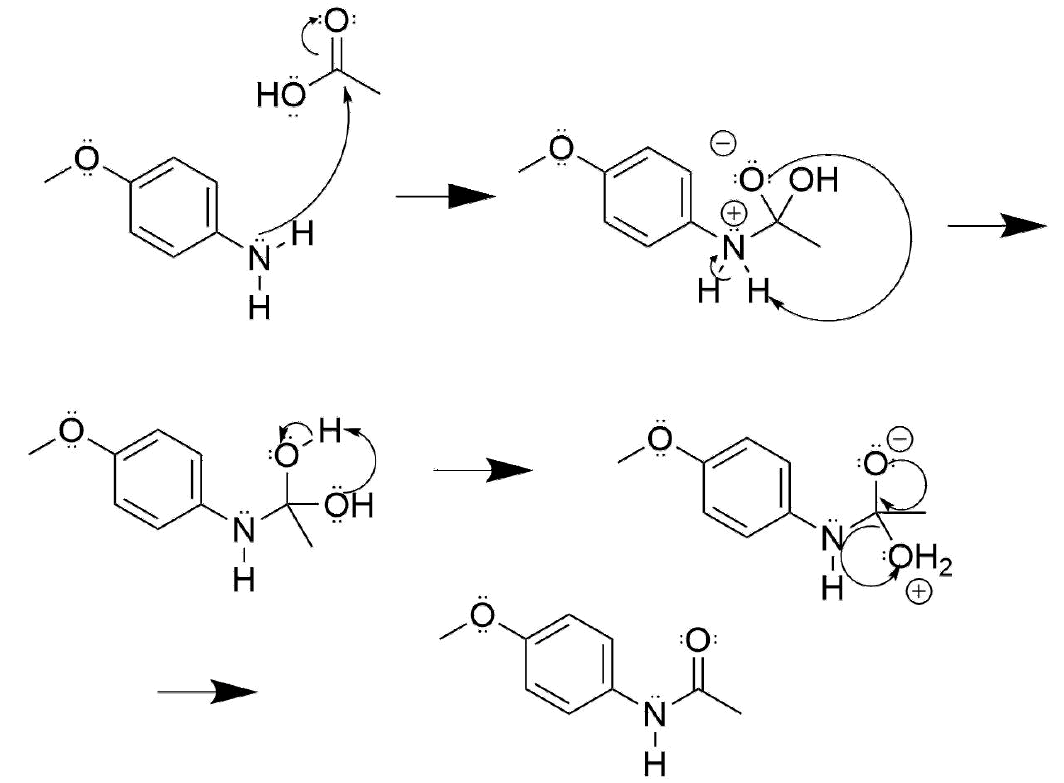
- Answer
-
Draw the product of the reaction between these two molecules.
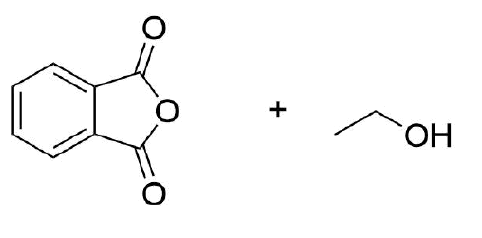
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

