16.5: Photochemical Smog
- Page ID
- 434971
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how photochemical smog is formed.
- Describe how industrial smog is formed.
- Identify harmful effects of smog.
Smog is a mixture of air pollutants (sulfur dioxide, nitrogen oxides, ozone, and particulates) that often form over urban areas as a result of fossil fuel combustion. Thus, smog is a byproduct of modern industrialization. Due to industry and the number of motor vehicles, it is more of a problem in large cities that have a warm, sunny, and dry climate. The term was coined from the terms “smoke” and “fog” referring to a brownish haze that pollutes the air, greatly reducing visibility and making it difficult for some people to breathe (Figure \(\PageIndex{1}\) and \(\PageIndex{2}\)). There are two main types of smog: photochemical and industrial smog. Photochemical smog is formed when sunlight drives chemical reactions between primary pollutants from automobiles and normal atmospheric compounds. The product is a mix of over 100 different chemicals with the most abundant being ground-level ozone. Industrial smog is produced primarily by the burning of fossil fuels which produces carbon dioxide (from complete combustion), carbon monoxides (from partial combustion), sulfur, and mercury. The sulfur reacts with other chemicals in the atmosphere producing several sulfur compounds including sulfur dioxide. These compounds along with particulate material make up industrial smog.


Formation of Photochemical Smog
Photochemical smog occurs in dry, stagnant air masses, usually stabilized by a temperature inversion (which traps gases close to the surface of the Earth as seen in Figure \(\PageIndex{1}\)), that are subjected to intense sunlight. The driving energy force behind smog formation is electromagnetic radiation from the sunlight with a wavelength at around 400 nm or less. This is in the ultraviolet region, just shorter than the lower limit for visible light. Energy absorbed by a molecule from this radiation can result in the formation of active species, thus initiating photochemical reactions.
Smog is produced in a series of chain reactions. There are two different series of chemical reactions which can take place in the formation of photochemical smog. The first involves only nitrogen oxides formed by combustion reactions.
Step 1: People begin driving in the morning, nitrogen reacts with oxygen in the extreme temperatures of the combustion engine.
\[{\mathrm N}_2\;+\:{\mathrm O}_2\;\rightarrow\;2\;\mathrm{NO}\]
Step 2: After a few hours, NO combines with O2 in another combustion reaction.
\[2\;\mathrm{NO}\;+\:{\mathrm O}_2\;\rightarrow\;2\;{\mathrm{NO}}_2\]
Step 3: Nitrogen dioxide absorbs light energy (hv), reforming NO and generating a highly reactive oxygen atom. The NO can then go back to react with more O2 in step 2 to continue cycling through the reaction or it can be used in later reactions.
\[{\mathrm{NO}}_2\;+\:h\nu\;\rightarrow\;\mathrm{NO}\;+\:\mathrm O\]
Step 4: In sunlight, the atomic oxygen combines with oxygen gas to form ozone.
\[\mathrm O\:+\:{\mathrm O}_2\;\rightarrow\;{\mathrm O}_3\]
Step 5: The ozone is consumed by NO to produce nitrogen dioxide and O2. Again, these products are able to be reused in earlier steps of the reaction to continue the cycle.
\[\mathrm{NO}\:+\:{\mathrm O}_3\;\rightarrow\;{\mathrm{NO}}_2\;+\:{\mathrm O}_2\]
These reactions result in the accumulation of various nitrogen oxides and some ozone which have the health and environmental effects discussed previously in Section 16.4. However, NO and NO2 can also enter into a second cycle of chemical reactions by reacting with volatile organic compounds (VOCs, shown as R) and oxygenated organic and inorganic compounds (ROx) instead of ozone to form a variety of other volatile compounds.
\[{\mathrm{NO}}_2\:+\:\mathrm R\;\rightarrow\;\mathrm{oxygenated}\;\mathrm{products}\]
\[\mathrm{NO}\:+\:{\mathrm{RO}}_{\mathrm x}\;\rightarrow\;{\mathrm{NO}}_2\;+\;\mathrm{other}\;\mathrm{products}\]
Because the nitrogen oxides react with VOCs in this second series of reactions, ozone is no longer broken down in the first cycle. Instead, ozone is allowed to build up. The accumulation of ozone and volatile organic compounds, along with the energy from the sun, forms the brown, photochemical smog seen on hot, sunny days (Figure \(\PageIndex{3}\)).

Because of the wide variety of VOCs present in the atmosphere, numerous noxious products are generated. As already stated, one of the main ones of these is ozone, O3, and it is the single species most characteristic of smog. Another class of materials formed with smog consists of oxygen-rich organic compounds containing nitrogen of which peroxyacetyl nitrate, PAN,
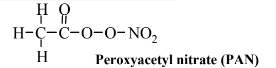
is the most common example. This compound, and ones similar to it, is a potent oxidizer and highly irritating to eyes and mucous membranes of the respiratory tract. Also associated with smog are aldehydes, which are irritants to eyes and the respiratory tract. The simplest aldehyde, and one commonly found in smoggy atmospheres, is formaldehyde:

Harmful Effects of Smog
Smog adversely affects human health and comfort as well as plants, materials, and atmospheric quality. Ozone is the smog constituent that is generally regarded as being most harmful to humans, plants, and materials, although other oxidants and some of the noxious organic materials, such as aldehydes, are harmful as well. These effects may be especially pronounced for individuals undergoing vigorous exercise because of the large amounts of air that they inhale. On smoggy days, air pollution alerts may advise against exercise and outdoor activities.
Plants are harmed by exposure to nitrogen oxides, ozone, and peroxyacetyl nitrate (PAN, see above), all species present in a smoggy atmosphere. PAN is the most harmful of these constituents, damaging younger plant leaves, especially. Some plant species, including sword-leaf lettuce, black nightshade, quickweed, and double-fortune tomato, are extremely susceptible to damage by chemicals in smog and are used as bioindicators of the presence of smog. Costs of crop and orchard damage by smog run into millions of dollars per year in areas prone to this kind of air pollution, such as southern California.
Materials that are adversely affected by smog are generally those that are attacked by oxidants. The best example of such a material is rubber, especially natural rubber, which is attacked by ozone. Indeed, the hardening and cracking of natural rubber has been used as a test for atmospheric ozone.
Visibility-reducing atmospheric aerosol particles are the most common manifestation of the harm done to atmospheric quality by smog. The smog-forming process occurs by the oxidation of organic materials in the atmosphere, and carbon-containing organic materials are the most common constituents of the aerosol particles in an atmosphere afflicted by smog. Conifer trees (pine and cypress) and citrus trees are major contributors to the organic hydrocarbons that are precursors to organic particle formation in smog.
Industrial Smog
Industrial smog or London-type smog is mainly a product of burning large amounts of high sulfur coal (Figure \(\PageIndex{4}\)). Clean air laws passed in 1956 have greatly reduced smog formation in the United Kingdom; however, in other parts of the world London-type smog is still very prevalent. The main constituent of London-type smog is soot; however, these smogs also contain large quantities of fly ash, sulfur dioxide, sodium chloride and calcium sulfate particles. If concentrations are high enough, sulfur dioxide can react with atmospheric hydroxide to produce sulfuric acid, which will precipitate as acid rain.
\({\mathrm{SO}}_2\;+\:\mathrm{OH}\;\rightarrow\;{\mathrm{HOSO}}_2\)
\({\mathrm{HOSO}}_2\;+\:{\mathrm O}_2\;\rightarrow\;{\mathrm{HO}}_2\;+\:{\mathrm{SO}}_3\)
\({\mathrm{SO}}_3\;+\:{\mathrm H}_2\mathrm O\;\rightarrow\;{\mathrm H}_2{\mathrm{SO}}_4\)
Concerns about the harmful effects of acid rain have led to strong pressure on industry to minimize the release of SO2. For example, coal-burning power plants now use SO2 “scrubbers,” which trap SO2 by its reaction with lime (CaO) to produce calcium sulfite dihydrate (CaSO3·2H2O) thus preventing its release into the atmosphere.

Summary
- Photochemical smog is a mixture of pollutants that are formed (mostly during the hot summer months) when nitrogen oxides and volatile organic compounds (VOCs) react with sunlight, creating a brown haze above cities.
- Smog is a serious problem in many cities and continues to harm human health and are especially harmful for senior citizens, children, and people with heart and lung conditions such as emphysema, bronchitis, and asthma.
- Industrial smog is a mixture of soot and other particulate matter, as well as sulfur dioxide gas.
- If concentrations are high enough, the sulfur dioxide in industrial smog can form sulfuric acid and acid rain.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
- Stanley Manahan (University of Missouri)
- Vicki MacMurdo (Anoka-Ramsey Community College)
- Wikipedia

