16.4: Air Pollution
- Page ID
- 434970
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define air pollution.
- Know the types and effects of major outdoor pollutants
- Know the sources of major outdoor pollutants
- Identify the different pollutant gases in automobile emissions
Air pollution refers to the introduction into the atmosphere of substances that have harmful effects on humans, other living organisms, and the environment. These substances can occur as solid particles, liquid droplets or gases. Air pollution can result from natural processes such as dust storms, forest fires, and volcanic eruptions, or from human activities such as biomass burning, vehicular emissions, mining, agriculture, and industrial processes. Improved technology and government policies have helped reduce most types of outdoor air pollution in many industrialized countries, including the United States, in recent decades. However, outdoor air quality is still a problem in less industrialized nations, especially in megacities of rapidly industrializing nations such as China and India.
Pollutants are categorized into two major types based on how they originated: namely primary and secondary pollutants. Primary pollutants are those released directly from the source into the air in a harmful form. The primary pollutants that account for nearly all air pollution problems are carbon monoxide (58%), volatile organic compounds (VOCs, 11%), nitrogen oxides (15%), sulfur dioxides (13%), and particulate material (3%). Secondary pollutants are produced through reactions between primary pollutants and normal atmospheric compounds. For example, ground-level ozone forms over urban areas through reactions powered by sunlight between primary pollutants (oxides of nitrogen) and other atmospheric gases such as VOCs.
Criteria pollutants
Under the US Clean Air Act, the Environmental Protection Agency (EPA) establishes air quality standards to protect public health and the environment. The EPA has set national air quality standards for six common air pollutants: carbon monoxide, ground-level ozone, nitrogen dioxide, sulfur dioxide, lead, and particulate matter (also known as particle pollution). The EPA calls these pollutants "criteria" air pollutants because it regulates them by developing human health-based and/or environmentally-based criteria (science-based guidelines) for setting permissible levels. The set of limits based on human health is called primary standards. Another set of limits intended to prevent environmental and property damage is called secondary standards. Of the six pollutants, particle pollution and ground-level ozone are the most widespread health threats.
- Carbon Monoxide (CO) is a colorless, odorless gas emitted from combustion processes, specifically the incomplete combustion of fuel. Nationally, and particularly in urban areas, the majority of CO emissions to ambient air occurs when something is burned. The greatest sources of CO to outdoor air are cars, trucks and other vehicles or machinery that burn fossil fuels. A variety of items in your home such as unvented kerosene and gas space heaters, leaking chimneys and furnaces, and gas stoves also release CO and can affect air quality indoors.
- Ground-level ozone (O3) is a colorless gas with a slightly sweet odor that is not emitted directly into the air, but is created by the interaction of sunlight, heat, oxides of nitrogen (NOx) and volatile organic compounds (VOCs). Ozone is likely to reach unhealthy levels on hot sunny days in urban environments. Emissions from industrial facilities and electric utilities, motor vehicle exhaust, gasoline vapors, and chemical solvents are some of the major sources of NOx and VOCs. Ozone can be transported long distances by wind, so even rural areas can experience high ozone levels.
- Nitrogen dioxide (NO2) is one of a group of highly reactive gases known as "oxides of nitrogen," or "nitrogen oxides (NOx)." Other nitrogen oxides include nitrous oxide (N2O, dinitrogen monoxide), nitric oxide (NO, nitrogen monoxide), and nitric acid. NO2 is a yellowish-brown to reddish-brown foul-smelling gas that is a major contributor to smog and acid rain. Nitrogen oxides result when atmospheric nitrogen and oxygen react at the high temperatures created by combustion engines. Most emissions in the U.S. result from combustion in vehicle engines, electrical utilities, and industrial combustion. They may also be produced by natural sources such as lightning. Nitrous oxide is generated by bacteria and its release is one of the ways in which chemically-fixed nitrogen in the biosphere is returned to the atmosphere.
- Sulfur dioxide (SO2) is one of a group of highly reactive gases known as “oxides of sulfur.” The largest sources of SO2 emissions are from fossil fuel combustion at power plants (73%) and other industrial facilities (20%). Smaller sources of SO2 emissions include industrial processes such as extracting metals from their ores, and the burning of high sulfur containing fuels by locomotives, large ships, and non-road equipment. They may also be directly released by volcanoes or formed by the oxidation of H2S and dimethyl sulfide, (CH3)2S, emitted by bacteria and marine organisms, respectively.
- Lead (Pb) is a metal found naturally in the environment as well as in manufactured products. The major sources of lead emissions have historically been from fuels in motor vehicles (such as cars and trucks) and industrial sources. As a result of EPA's regulatory efforts to remove lead from gasoline, emissions of lead from the transportation sector dramatically declined by 95 percent between 1980 and 1999, and levels of lead in the air decreased by 94 percent during that time period. The major sources of lead emissions today are ore and metal processing and piston-engine aircraft operating on leaded aviation gasoline. Today, the highest levels of lead in air are usually found near lead smelters.
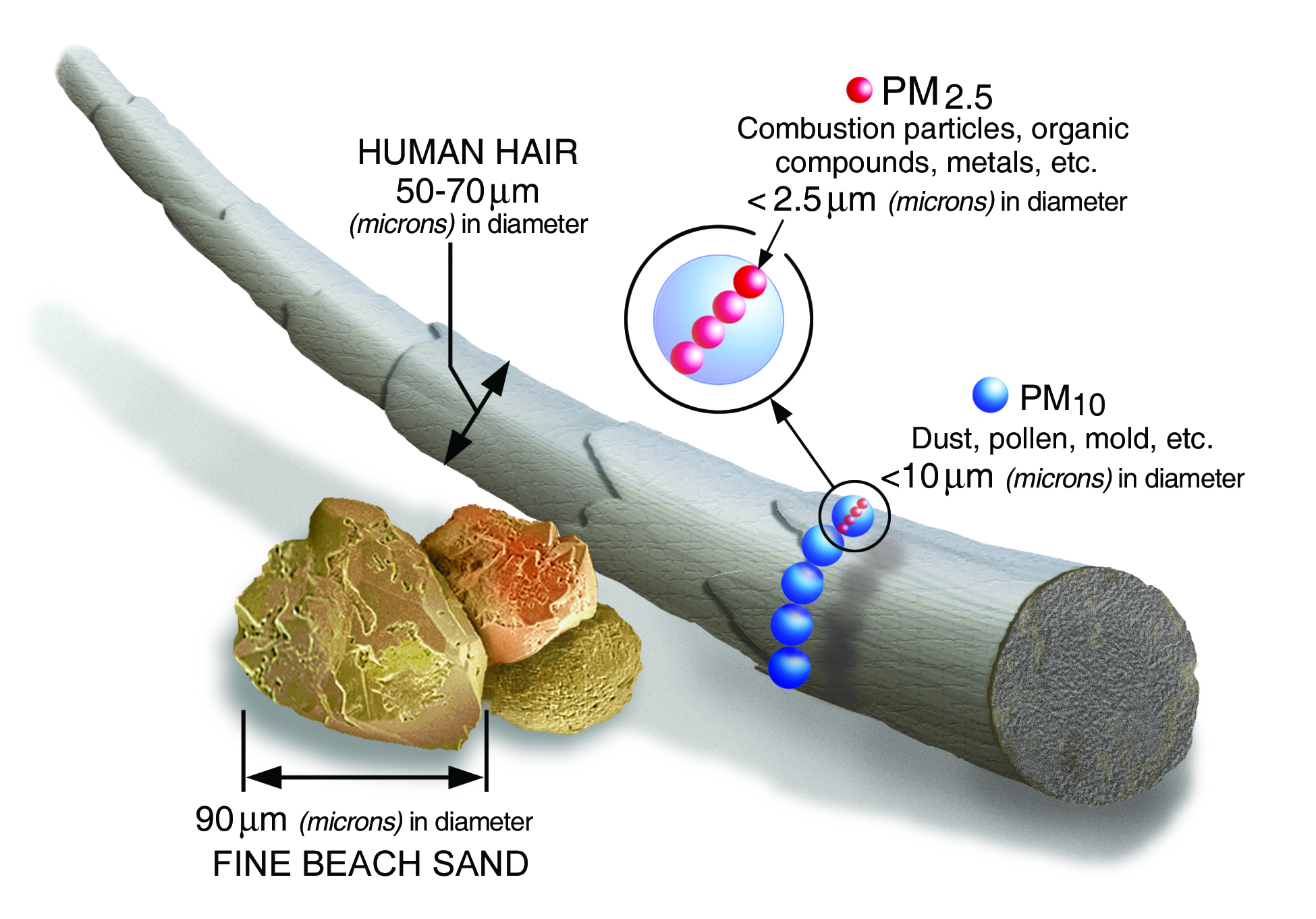
- Particulate material (PM), sometimes known simply as “particulates” refers to solid particles and liquid droplets suspended in the air we breathe. Particulate pollution is made up of a variety of components, including acids (nitrates and sulfates), organic chemicals, metals, soil or dust particles, and allergens (pollen and mold spores). The size of the particles is directly linked to their potential for causing health problems. Particles that are 10 micrometers in diameter or smaller generally pass through the throat and nose and enter the lungs. EPA groups these into two types: "inhalable coarse particles" with diameters between 2.5 and 10 micrometers and "fine particles" with diameters that are 2.5 micrometers and smaller. How small is 2.5 micrometers? Think about a single hair from your head. The average human hair is about 70 micrometers in diameter – making it 30 times larger than the largest fine particle (Figure \(\PageIndex{2}\)). Our respiratory systems are equipped to filter larger particles out of the air once it is inhaled. However, the lungs are vulnerable to both coarse particles (PM10), and fine particles (PM2.5). These can slip past the respiratory system's natural defenses and get deep into the lungs and some may even get into the bloodstream. Coarse particles come from road dust while fine particles come from combustion processes.
Volatile Organic Compounds
Volatile organic compounds (VOCs) are carbon-containing chemicals emitted as gases from natural and human-made sources. The largest natural source of VOCs is plants. They can also be emitted by bacteria in the guts of termites and ruminant animals. These compounds are generally oxidized to carbon monoxide and carbon dioxide in the atmosphere. VOCs are of great concern because they are precursors for the formation of ozone, a secondary air pollutant.
A large number of synthetic organic chemicals such as benzene, toluene, formaldehyde, vinyl chloride, chloroform, and phenols are widely used as ingredients in countless household products. Paints, paint strippers, varnishes, as well as many cleaning, disinfecting, cosmetic, degreasing, and hobby products all contain VOCs. Fuels are also made up of organic chemicals. All of these products can release organic compounds while you are using them and, to some degree, when they are stored. The “new car smell” characteristic of new cars is from a complex mix of dozens of VOCs. In addition, concentrations of many VOCs are consistently higher indoors (up to ten times higher) than outdoors. They are often held responsible for sick building syndrome, an illness resulting from indoor pollution in which the specific cause is not identified.
Toxic pollutants
Toxic air pollutants, also known as hazardous air pollutants, are those pollutants that are known or suspected to cause cancer or other serious health effects, such as reproductive effects or birth defects, or adverse environmental effects. Examples of toxic air pollutants include benzene, which is found in gasoline; perchloroethylene, which is emitted from some dry cleaning facilities; methylene chloride, which is used as a solvent and paint stripper by a number of industries; and others such as dioxin, asbestos, toluene, and metals such as cadmium, mercury, chromium, and lead compounds.
Most air toxics originate from human-made sources, including vehicles, factories, refineries, and power plants, as well as indoor sources such as some building materials and cleaning solvents. Some air toxics are also released from natural sources such as volcanic eruptions and forest fires. Exposure to air toxics is mainly through breathing but some toxic air pollutants such as mercury can deposit onto soils or surface waters where they are taken up by plants and ingested by animals and are eventually magnified up through the food chain. Like humans, animals may experience health problems if exposed to sufficiently high quantities of air toxics over time.
Effects of Air Pollution on Human Health and the Environment
The World Health Organization (WHO) and other international agencies recognize air pollution as a major threat to human health. Numerous scientific studies have linked air pollution to a variety of health problems (Table \(\PageIndex{1}\)) including aggravation of respiratory and cardiovascular diseases; decreased lung function; increased frequency and severity of respiratory symptoms such as difficulty breathing and coughing; increased susceptibility to respiratory infections; effects on the nervous system, including the brain, such as IQ loss and impacts on learning, memory, and behavior; cancer; and premature death. Immediate effects of air pollution may show up after a single exposure or repeated exposures. Other health effects may show up either years after exposure has occurred or only after long or repeated periods of exposure.
Immediate effects of air pollution include irritation of the eyes, nose, and throat, headaches, dizziness, and fatigue. Such immediate effects are usually short-term and treatable. Sometimes the treatment is simply eliminating the person's exposure to the source of the pollution, if it can be identified. Symptoms of some diseases, including asthma, hypersensitivity pneumonitis, and humidifier fever, may also show up soon after exposure to some indoor air pollutants.
Source: www.epa.gov
The likelihood of immediate reactions to air pollutants depends on several factors. Age and preexisting medical conditions are two important influences. Some sensitive individuals appear to be at greater risk for air pollution-related health effects, for example, those with pre-existing heart and lung diseases (such as heart failure/ischemic heart disease, asthma, emphysema, and chronic bronchitis), diabetics, older adults, and children. In other cases, whether a person reacts to a pollutant depends on individual sensitivity, which varies tremendously from person to person. Some people can become sensitized to biological pollutants after repeated exposures, and it appears that some people can become sensitized to chemical pollutants as well.
In addition to posing a threat to human health, many air pollutants have damaging effects on the environment as a whole. Both nitrogen oxides and sulfur dioxide react with gases in the atmosphere and then combine with water droplets to form acid rain. As will be seen in Section 16.5, ground-level ozone is a main ingredient in "smog" that occurs in urban areas and results in lowers visibility. The haziness can be aggravated by particulate matter formed by nitrogen oxides as well. Ozone can harm sensitive vegetation and ecosystems such as forests, parks and wildlife refugees, particularly during the growing season. Sulfur dioxide causes leaf necrosis (death of leaf tissue) and chlorosis (bleaching or yellowing of green leaves). Health effects seen in humans with pollutants such as lead can also be observed in animals in nature.
Sources of Pollution
As already mentioned, air pollution is caused by the introduction of substances that have harmful effects on biological systems into the atmosphere. It can be introduced by natural processes such as dust storms, forest fires, volcanic eruptions, or biogenics (emissions from vegetation and soil bacteria). However, there is little that we can do to reduce these types of natural emissions. Instead, we will focus on pollution that is anthropogenic, the result of human activities, as this is something that we can influence.
Outdoor pollutants can come from mobile or stationary sources (Figure \(\PageIndex{2}\)). Mobile sources of air pollutants move from place to place while emitting pollutants. Examples of mobile sources include vehicles, aircrafts, ships, and trains. Stationary sources have a fixed location. Examples include power plant smokestacks, industrial processes, burning, residential heating, construction sites, farmlands and surface mines among others. Stationary sources can be further broken down into point and nonpoint sources. Point sources refer to larger emitters at a fixed location, such as an industrial facility. Nonpoint sources tend to be abundant and smaller in magnitude, such as residential heating.


Much of the pollution from human activities is due to the burning of fossil fuels. We utilize fuel combustion to heat homes, generate electricity, and propel a variety of vehicles. Exhaust gas or flue gas is emitted as a result of the combustion of fuels such as natural gas, gasoline, petrol, biodiesel blends, diesel fuel, fuel oil, or coal. It is a major component of both motor vehicle (and stationary internal combustion engine) emissions. Motor vehicle emissions contribute to air pollution and are a major ingredient in the creation of smog in some large cities. A 2013 study by MIT indicates that 53,000 early deaths occur per year in the United States alone because of vehicle emissions. In addition to the products of combustion, exhaust gases can also include unreacted gasoline vapors. Many fuels such as coal and wood also directly release particulate matter into the atmosphere.
There are anthropogenic sources of pollution that do not come from combustion reactions. Some of these pollutants are by-products of industrial processes while others are the actual chemicals themselves. Many of these are volatile organic compounds (VOCs) which are carbon containing compounds used as ingredients in many household products. These compounds can vaporize and thus enter into the atmosphere. Others pollutants are compounds such as asbestos or heavy metals like mercury and lead which disperse as particulate matter and can deposit onto soils and surface waters to be taken up by plants and enter into the food chain. Emissions of the most prevalent pollutants are monitored by the government (Figure \(\PageIndex{3}\)).
Summary
- Air pollution refers to the introduction, into the atmosphere, of substances that have harmful effects on humans, other living organisms, and the environment either as solid particles, liquid droplets or gases.
- Major sources of air pollution are gas emissions from fossil-fueled vehicles (and other processes which burn fossil fuels) and their reaction products (CO, NOx, ozone) as well as particulate matter, SO2, and VOCs.
- Ozone is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC).
- Examples of toxic air pollutants include benzene, which is found in gasoline; perchloroethylene, which is emitted from some dry cleaning facilities; methylene chloride, which is used as a solvent and paint stripper by a number of industries; and others such as dioxin, asbestos, toluene, and metals such as cadmium, mercury, chromium, and lead compounds.
- Numerous scientific studies have linked air pollution to a variety of health problems.
Contributors and Attributions
- Libretext: Introduction to Environmental Science (Zendher et al.)
- TextMap: General Chemistry (Averill and Eldredge)
- US Environmental Protection Agency
- Wikipedia
- Vicki MacMurdo (Anoka-Ramsey Community College)
- Stanley E. Manahan (University of Missouri)



