15.8: pH and pOH Calculations
- Page ID
- 359221
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Define pH and pOH.
- Determine the pH of acidic and basic solutions.
- Determine the hydronium ion concentration and pOH from pH.
The pOH scale
In the previous section, the pH was defined as the negative logarithm of the hydronium ion concentration:
\[\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]\]
It is likely you have only heard of the pH scale. However, there is a pH counterpart called the pOH (the "power of the hydroxide ion"), which is defined as the negative logarithm of the hydroxide ion concentration:
\[\mathrm{pOH}=-\log\left[\mathrm{OH}^-\right]\]
For aqueous solutions at 25°C, the sum of the pH and pOH is always 14.00:
\[\mathrm{pH}\;+\;\mathrm{pOH}\;=14.00\]
While its derivation is beyond the scope of this text, Equation \(\PageIndex{3}\) is a mathematical extension of the relationship between the \(\left[{\mathrm H}_3\mathrm O^+\right]\) and the \(\left[\mathrm{OH}^-\right]\), established in Section 15.6, in which their product is 1.0 × 10–14 at 25°C:
\({\color[rgb]{0.8, 0.0, 0.0}\left[{\mathrm H}_3\mathrm O^+\right]}{\color[rgb]{0.0, 0.0, 0.8}\left[\mathrm{OH}^-\right]}=1.0\times10^{-14}\)
Simple pH and pOH Calculations
In pure water, we have already established that \(\left[{\mathrm H}_3\mathrm O^+\right]=\left[\mathrm{OH}^-\right]=10^{-7}\;\mathrm M\). Substituting \(\left[\mathrm{OH}^-\right]=10^{-7}\;\mathrm M\) into the equation, \(\mathrm{pOH}=-\log\left[\mathrm{OH}^-\right]\):
\(\mathrm{pOH}=-\log\left[10^{-7}\right]=-\left(-7\right)=7\)
Thus, both the pH = 7 and pOH = 7 for pure water. This makes sense, since the pH and pOH should sum to 14, as shown in Equation \(\PageIndex{3}\) above. As previously noted, temperature matters. However, if the temperature is not cited, a temperature of 25°C (room temperature) may be assumed.
✅ Example \(\PageIndex{1}\)
What is the pH of these solutions?
- pOH = 5.55
- \(\left[{\mathrm H}_3\mathrm O^+\right]\) = 10–11 M
- \(\left[\mathrm{OH}^-\right]\) = 10–8 M
Solution
- From Equation \(\PageIndex{3}\), pH + pOH = 14.00. Therefore, pH = 14.00 – pOH = 14.00 – 5.55 = 8.45.
- From Equation \(\PageIndex{1}\), \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]=-\log\left(10^{-11}\right)=-(-11)=11\)
- From Equation \(\PageIndex{2}\), \(\mathrm{pOH}=-\log\left[\mathrm{OH}^-\right]\;=\;-\log\left(10^{-8}\right)=-(-8)=8\). From Equation \(\PageIndex{3}\), pH + pOH = 14.00. Therefore, pH = 14.00 – pOH = 14.00 – 8 = 6.
✏️ Exercise \(\PageIndex{1}\)
What is the pOH of these solutions?
- \(\left[\mathrm{OH}^-\right]\) = 10–4 M
- \(\left[{\mathrm H}_3\mathrm O^+\right]\) = 10–12 M
- pH = 9.01
- Answer A
- pOH = 4
- Answer B
- pOH = 2
- Answer C
- pOH = 4.99
Figure \(\PageIndex{1}\) summarizes the relationships between \(\left[{\mathrm H}_3\mathrm O^+\right]\), \(\left[\mathrm{OH}^-\right]\), pH, and pOH. Note that the product of \(\left[{\mathrm H}_3\mathrm O^+\right]\) and \(\left[\mathrm{OH}^-\right]\) is always 1.0 × 10–14 M and that the sum of the pH and pOH is always 14.
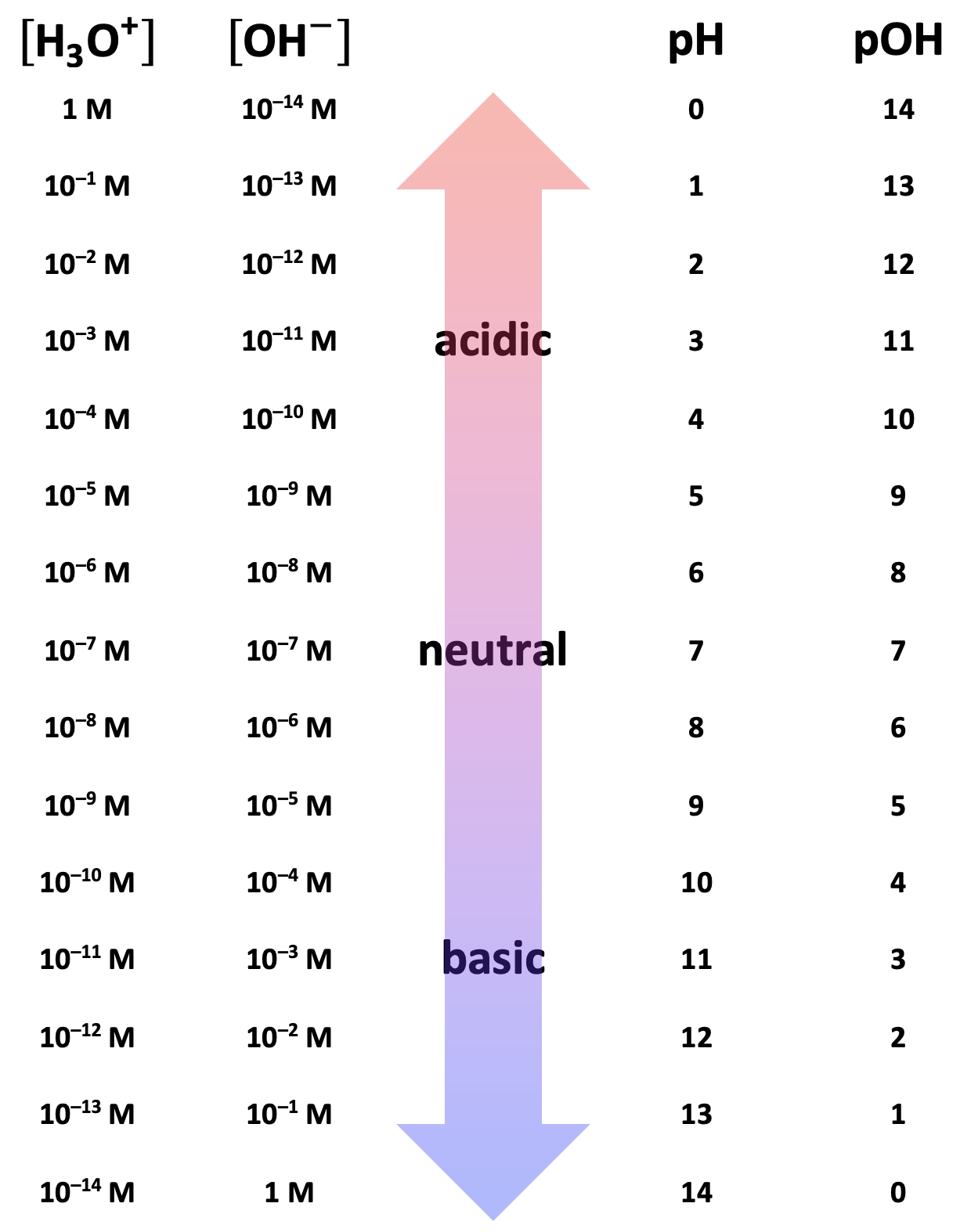
pH and pOH Calculations That Require a Calculator
Calculations more complex than the ones shown above likely require a calculator. It is impossible to provide instructions for every available model of calculator, but we will look at three different models. The large majority of calculators share one of the sequences used by the calculators shown in Figure \(\PageIndex{2}\).
Calculate the pH of the following solutions:
- \(\left[{\mathrm H}_3\mathrm O^+\right]\) = 5.6 × 10–5 M
- \(\left[{\mathrm H}_3\mathrm O^+\right]\) = 5.7 × 10–5 M
- \(\left[{\mathrm H}_3\mathrm O^+\right]\) = 5.7 × 10–4 M
Solution
- \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]=-\log\left(5.6\times10^{-5}\right)=4.2\underline518=\boxed{4.25}\)
- \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]=-\log\left(5.7\times10^{-5}\right)=4.2\underline441=\boxed{4.24}\)
- \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]=-\log\left(5.7\times10^{-4}\right)=3.2\underline441=\boxed{3.24}\)
The procedure for determining significant figures in pH calculations is described below. The method for using a calculator to calculate pH for Part A follows.
Look carefully at the \(\left[{\mathrm H}_3\mathrm O^+\right]\) for Parts A and B in Example \(\PageIndex{2}\) above. Each measurement has two significant figures and the uncertain digit is the second significant figure (last digit). Upon calculating the pH, we see in the Solution that the uncertain digit is the second digit after the decimal. When comparing the \(\left[{\mathrm H}_3\mathrm O^+\right]\) for Parts B and C, we notice that changing the exponent on the power of 10 changes the number that is in front of the decimal in the calculated pH.
Since each measurement can only have one uncertain digit, this leads us to conclude:
- The calculated pH will have the same number of decimal places as the number of significant figures in the \(\left[{\mathrm H}_3\mathrm O^+\right]\).
In other words, if the \(\left[{\mathrm H}_3\mathrm O^+\right]\) has only one significant figure, then the calculated pH will only have one decimal place. A similar statement may be made about the number of significant figures with regard to pOH and the \(\left[\mathrm{OH}^-\right]\):
- The calculated pOH will have the same number of decimal places as the number of significant figures in the \(\left[{\mathrm H}_3\mathrm O^+\right]\).
✍️ Using a Scientific Calculator to Calculate pH
To calculate the answer to Example \(\PageIndex{2}\) Part A:
On a TI-83, TI-84, or TI-30XIIS:
- \(\boxed{(-)}\;\boxed\log\;5.6\;\boxed{2\mathrm{nd}}\;\boxed{\mathrm{EE}}\;\boxed{(-)}\;5\;\boxed{\;\;)\;\;}\)
- The EE function is located above the \(\boxed{{}^\;\;,{}^\;\;}\) button on a TI-83 and TI-84. It is located above the \(\boxed{\;x^{-1}\;}\) button on a TI-30XIIS.
- At this point your display should look like this: \(-\log\left(5.7\mathrm E-5\right)\) and you can press the \(\boxed{\mathrm{Enter}}\) button.
- An answer of 4.2518... will be displayed and need to be rounded to the proper number of significant figures.
On a Casio fx-61F:
- \(5.6\;\boxed{\mathrm{EXP}}\;5\;\;\boxed{+/-}\;\boxed\log\;\boxed{+/-}\;\boxed{\;=\;}\)
- An answer of 4.2518... will be displayed and need to be rounded to the proper number of significant figures.
✅ Example \(\PageIndex{3}\)
Calculate the pH of the following solutions:
- 0.10 M HCl
- 0.0044 M Ca(OH)2
Solution
| Steps for Problem Solving | 0.10 M HCl | 0.0044 M Ca(OH)2 |
|---|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: HCl = 0.10 M Find: pH |
Given: Ca(OH)2 = 0.0044 M Find: pH |
List known relationship(s). |
HCl is a strong acid. Therefore: \(\left[{\mathrm H}_3\mathrm O^+\right]\;=\;\left[\mathrm{acid}\right]\) \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]\) |
Ca(OH)2 is a strong base. Therefore: \(\left[\mathrm{OH}^-\right]=\left[\mathrm{base}\right]\times\mathrm{number}\;\mathrm{of}\;\mathrm{OH}^-\;\mathrm{ions}\;\mathrm{in}\;\mathrm{the}\;\mathrm{chemical}\;\mathrm{formula}\) \(\mathrm{pOH}=-\log\left[\mathrm{OH}^-\right]\) \(\mathrm{pH}\;+\;\mathrm{pOH}\;=14.00\) |
| Plan the problem. | Calculate the pH by plugging the \(\left[{\mathrm H}_3\mathrm O^+\right]\) into the equation. | Calculate the \(\left[\mathrm{OH}^-\right]\). Calculate the pOH by plugging the \(\left[\mathrm{OH}^-\right]\) into the equation. Calculate the pH by rearranging and plugging in the pOH: pH = 14.00 – pOH |
| Calculate the answers. | \(\mathrm{pH}=-\log\left[{\mathrm H}_3\mathrm O^+\right]=-\log\left(0.10\right)=\boxed{1.00}\) | \(\left[\mathrm{OH}^-\right]=0.0044\;\mathrm M\;\times\;2\;=\;0.0088\;\mathrm M\) \(\mathrm{pOH}=-\log\left[\mathrm{OH}^-\right]=-\log\left(0.0088\right)=2.06\) \(\mathrm{pH}=14.00-\mathrm{pOH}=14.00-2.06=\boxed{11.94}\) |
Think about your results. |
HCl is a strong acid. A pH = 1.00 would be very acidic, so the answer makes sense. | Ca(OH)2 is a strong base. A pH = 11.94 would be quite basic, so the answer makes sense. |
✏️ Exercise \(\PageIndex{2}\)
What is the pH of these solutions?
- 0.10 M NaOH
- 6.1 × 10–3 M HI
- \(\left[\mathrm{OH}^-\right]\) = 2.0 × 10–7 M
- Answer A
- pH = 13.00
- Answer B
- pH = 2.21
- Answer C
- pH = 7.30
Calculating Hydronium Concentration from pH
In some cases, the pH of a solution is known and the \(\left[{\mathrm H}_3\mathrm O^+\right]\) or concentration of the acid solution is needed. To convert pH to \(\left[{\mathrm H}_3\mathrm O^+\right]\), Equation \(\PageIndex{1}\) may be rearranged to solve for \(\left[{\mathrm H}_3\mathrm O^+\right]\). This involves taking the antilog (or inverse log) of the negative value of pH. Yeah, sounds complicated...so we'll spare you the details and show you the end result:
\[\left[{\mathrm H}_3\mathrm O^+\right]=10^{-\mathrm{pH}}\]
A similar equation exists to retrieve the \(\left[\mathrm{OH}^-\right]\) from the pOH:
\[\left[\mathrm{OH}^-\right]=10^{-\mathrm{pOH}}\]
Calculate the hydronium ion and hydroxide ion concentrations of a solution with a pH of 12.60.
Solution
| Steps for Problem Solving | |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: pH = 12.60 Find: \(\left[{\mathrm H}_3\mathrm O^+\right]\) = ? M; \(\left[\mathrm{OH}^-\right]\) = ? M |
| List known relationship(s). | \(\left[{\mathrm H}_3\mathrm O^+\right]=10^{-\mathrm{pH}}\) \(\mathrm{pH}\;+\;\mathrm{pOH}\;=14.00\) \(\left[\mathrm{OH}^-\right]=10^{-\mathrm{pOH}}\) |
| Plan the problem. | Find \(\left[{\mathrm H}_3\mathrm O^+\right]\) by plugging pH into the equation. Calculate the pOH by rearranging and plugging in the pH: pOH = 14.00 – pH Find \(\left[\mathrm{OH}^-\right]\) by plugging pOH into the equation. |
| Calculate the answers. | \(\left[{\mathrm H}_3\mathrm O^+\right]=10^{-\mathrm{pH}}=10^{-12.60}=\boxed{2.5\times10^{-13}\;\mathrm M}\) \(\mathrm{pOH}=14.00-\mathrm{pH}=14.00-12.60=1.40\) \(\left[\mathrm{OH}^-\right]=10^{-\mathrm{pOH}}=10^{-1.4}=\boxed{0.040\;\mathrm M}\) |
| Think about your results. | The \(\left[{\mathrm H}_3\mathrm O^+\right]\) and \(\left[\mathrm{OH}^-\right]\) should each have two significant figures, since the pH had two decimal places. A quick check shows that \(\left[{\mathrm H}_3\mathrm O^+\right]\left[\mathrm{OH}^-\right]=(2.5\times10^{-13})(0.040)=1.0\times10^{-14}\). |
✍️ Using a Scientific Calculator to Calculate \(\left[{\mathrm H}_3\mathrm O^+\right]\)
To calculate the \(\left[{\mathrm H}_3\mathrm O^+\right]\) in Example \(\PageIndex{4}\) above:
On a TI-83, TI-84, or TI-30XIIS:
- \(10\;\boxed{\;\;\hat{}\;}\;\;\boxed{\;\;(\;\;}\;\boxed{(-)}\;12.60\;\boxed{\;\;)\;\;}\)
- At this point your display should look like this: \(10\hat{}(-12.60)\) and you can press the \(\boxed{\mathrm{Enter}}\) button.
- An answer of 2.511886...E–13 will be displayed and will need to be rewritten in scientific notation and rounded to the proper number of significant figures (\(2.5\times10^{-13}\)).
On a Casio fx-61F:
- \(10\;\boxed{\mathrm{SHIFT}}\;\boxed{\;\mathrm x^{\mathrm y}\;}\;12.60\;\boxed{+/-}\;\boxed{\;=\;}\)
- An answer of 2.511886...–13 will be displayed and is very easily misinterpreted. It needs to be properly rewritten in scientific notation rounded to the proper number of significant figures (\(2.5\times10^{-13}\)).
- If moist soil has a pH of 7.84, find the \(\left[{\mathrm H}_3\mathrm O^+\right]\) and \(\left[\mathrm{OH}^-\right]\) of the soil sample.
- When lime (CaO) is added to this soil, its pH increases to 8.42. Find the new \(\left[{\mathrm H}_3\mathrm O^+\right]\) and \(\left[\mathrm{OH}^-\right]\) of the soil sample.
- When sulfur is added to this soil, its pH decreases to 6.57. What is the new \(\left[{\mathrm H}_3\mathrm O^+\right]\) and \(\left[\mathrm{OH}^-\right]\) of the sample?
- Answer A
-
\(\left[{\mathrm H}_3\mathrm O^+\right]\) = 1.4 × 10–8 M; \(\left[\mathrm{OH}^-\right]\) = 6.9 × 10–7 M
- Answer B
-
\(\left[{\mathrm H}_3\mathrm O^+\right]\) = 3.8 × 10–9 M; \(\left[\mathrm{OH}^-\right]\) = 2.6 × 10–6 M
- Answer C
-
\(\left[{\mathrm H}_3\mathrm O^+\right]\) = 2.7 × 10–7 M; \(\left[\mathrm{OH}^-\right]\) = 3.7 × 10–8 M
This page is shared under CK-12 and CC BY-NC-SA 4.0 licenses and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College), Peggy Lawson (Oxbow Prairie Heights School, funded by Saskatchewan Educational Technology Consortium), Marisa Alviar-Agnew, and Henry Agnew. Original sources: https://www.ck12.org/c/chemistry/ and https://openstax.org/details/books/chemistry-2e.






