15.4: Acids and Bases Defined
- Page ID
- 289466
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Identify an Arrhenius acid and an Arrhenius base.
- Identify a Brønsted-Lowry acid and a Brønsted-Lowry base.
- Identify conjugate acid-base pairs in an acid-base reaction.
There are three major classifications of substances known as acids or bases. The theory developed by Svante Arrhenius in 1883, the Arrhenius definition, states that an acid produces H+ in solution and a base produces OH–. Later, two more sophisticated and general theories were proposed. These theories are the Brønsted-Lowry and Lewis definitions of acids and bases. This text considers the Arrhenius and Brønsted-Lowry theories.
The Arrhenius Theory of Acids and Bases
In 1884, the Swedish chemist Svante Arrhenius proposed two specific classifications of compounds, termed acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. According to Arrhenius, an acid is a compound that increases the concentration of hydrogen ions, H+, when dissolved in water. Over time, this definition has slightly evolved. Today, an Arrhenius acid is often thought of as a compound that increases the concentration of hydronium ions, H3O+, when dissolved in water.
If you're thinking, "Wait a minute! I have never heard of hydronium ions," you are correct at least as far as chemical nomenclature is concerned. This is because hydronium ions are not found in compounds. Hydronium ions are only found in aqueous solutions of acids. In other words, you will never see a compound such as hydronium sulfate or hydronium chloride. So what, exactly, are hydronium ions? Read on:
A hydrogen ion, H+, represents a hydrogen atom that has lost its only electron. 99% of hydrogen atoms are comprised of the isotope hydrogen-1, \({}_1^1\mathrm H\), meaning that 99% hydrogen atoms have no neutrons in their nucleus. Because of this, an H+ ion is often thought of as a single proton, p+. Due to their tiny size in comparison to the size of an atom, individual protons represent an extremely concentrated charge. As a result, they attract the lone pairs of neighboring water molecules.
A proton in aqueous solution may be surrounded by more than one water molecule, leading to formulas like H5O2+ or H9O4+ rather than H3O+. It is simpler, however, to use H3O+ to represent the hydronium ion.
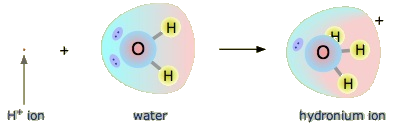
To represent the process using chemical equations, consider HCl (aq). The HCl (aq) releases H+ ions:
\[\mathrm{HCl}\;(aq)\;\rightarrow\;\mathrm H^+\;(aq)\;+\;\mathrm{Cl}^-\;(aq)\]
The H+ ions combine with H2O to form H3O+ ions as shown above:
\[\mathrm H^+\;(aq)\;+\;{\mathrm H}_2\mathrm O\;(l)\;\rightarrow\;{\mathrm H}_3\mathrm O^+\;(aq)\]
Summing Equation \(\PageIndex{1}\) and Equation \(\PageIndex{2}\) yields an equation that shows how hydronium ions are formed when an acid such as HCl is dissolved in water:
\[\mathrm{HCl}\;(aq)\;+\;{\mathrm H}_2\mathrm O\;(l)\;\rightarrow\;{\mathrm H}_3\mathrm O^+\;(aq)\;+\;\mathrm{Cl}^-\;(aq)\]
An Arrhenius base is a compound that increases the concentration of hydroxide ions, OH–, when dissolved in water. The dissociation of NaOH in water is represented by the following equation:
\[\mathrm{NaOH}\;(s)\;\xrightarrow{{\mathrm H}_2\mathrm O}\;\mathrm{Na}^+\;(aq)\;+\;\mathrm{OH}^-\;(aq)\]
In this reaction, sodium hydroxide (NaOH) dissociates into sodium ions, Na+, and hydroxide ions, OH–, when dissolved in water, thereby releasing OH– ions into solution.
⚓️ Arrhenius Theory of Acids and Bases
- An Arrhenius acid is a compound that increases the concentration of hydronium ions, H3O+, when dissolved in water.
- An Arrhenius base is a compound that increases the concentration of hydroxide ions, OH–, when dissolved in water.
The Brønsted-Lowry Theory of Acids and Bases
In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases, ones that focus on proton transfer. As described above, an H+ ion may be thought of as a single proton, p+. A Brønsted-Lowry acid is any species that donates a proton (H+) to another species, while a Brønsted-Lowry base is any species that accepts a proton from another species. In short, a Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
Let's use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH− (aq)
What has happened in this reaction is that the original water molecule has donated a hydrogen ion to the original ammonia molecule, which in turn has accepted the hydrogen ion from the water molecule. We can illustrate this as follows:
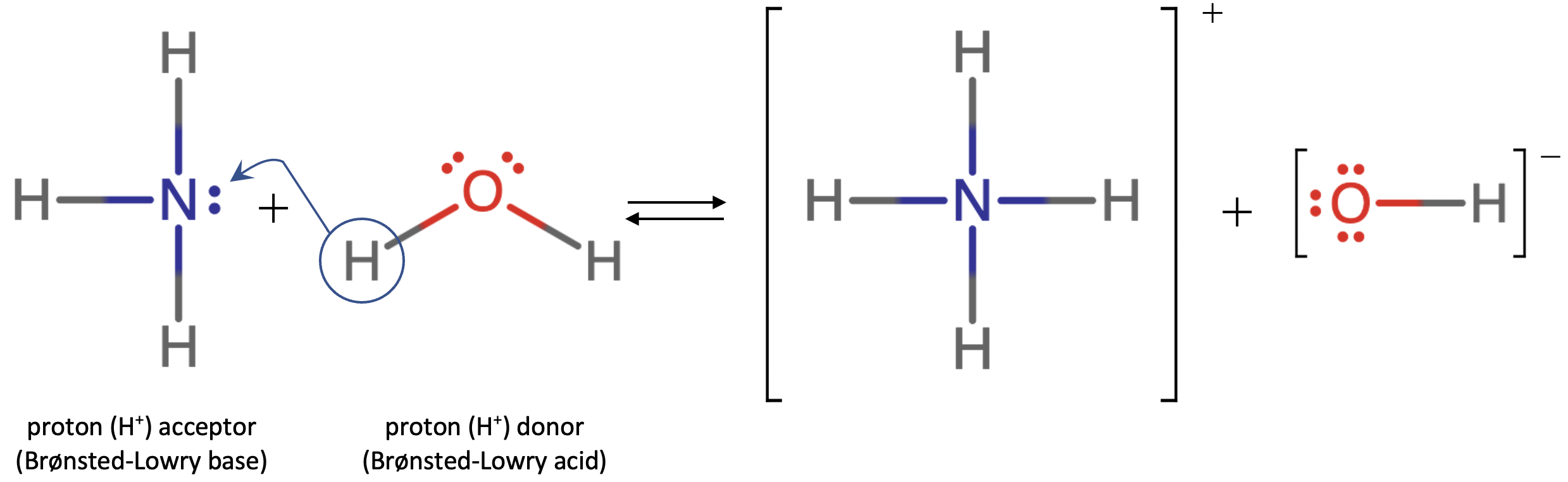
Because the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule – which accepts the hydrogen ion – is the Brønsted-Lowry base. Thus, ammonia acts as a base in both the Arrhenius sense (since it produces OH– ions when dissolved in water) and the Brønsted-Lowry sense (since it acts as a proton acceptor).
What about hydrochloric acid? Is an Arrhenius acid like HCl still an acid in the Brønsted-Lowry sense? Yes. Once again, the reaction that occurs when HCl is dissolved in H2O looks like this:
HCl (aq) + H2O (l) ⇌ H3O+ (aq) + Cl– (aq)
This process may be depicted using Lewis dot diagrams:
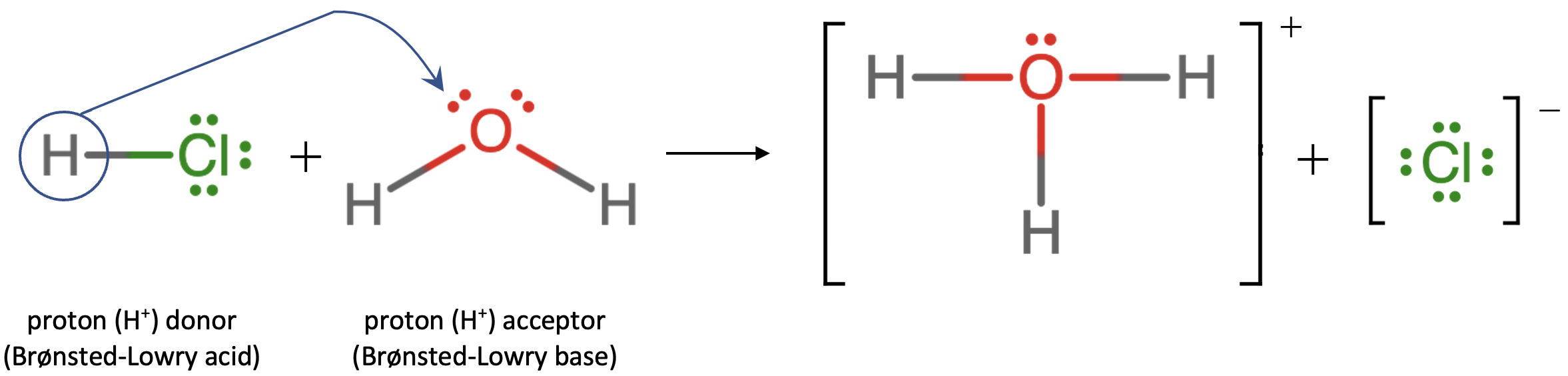
This equation shows that a hydrogen ion is transferred from the HCl molecule to the H2O molecule yielding chloride ions and hydronium ions. As the proton donor, HCl acts as a Brønsted-Lowry acid; as a proton acceptor, H2O is a Brønsted-Lowry base. In other words, HCl is both an Arrhenius acid and a Brønsted-Lowry acid. Moreover, by the Brønsted-Lowry definitions, H2O is a base in the formation of aqueous HCl.
The Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base. However, the Arrhenius definition would not have labeled H2O a base for the reaction shown in Figure \(\PageIndex{2}\), nor would it have labeled H2O as an acid in Figure \(\PageIndex{1}\).
⚓️ Brønsted-Lowry Theory of Acids and Bases
- A Brønsted-Lowry acid is a proton (H+) donor.
- A Brønsted-Lowry base is a proton (H+) acceptor.
Note: All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. However, not all Brønsted-Lowry acids and bases are Arrhenius acids and bases.
✅ Example \(\PageIndex{1}\)
Aniline (C6H5NH2) is slightly soluble in water. It has a nitrogen atom that can accept a hydrogen ion from a water molecule, just like the nitrogen atom in ammonia does. Write the chemical equation for this reaction and identify the Brønsted-Lowry acid and base.
Solution
C6H5NH2 and H2O are the reactants. When C6H5NH2 accepts a proton from H2O, it gains an extra H and a positive charge and leaves an OH− ion behind. The reaction may be written as follows:
C6H5NH2 (aq) + H2O (l) ⇌ C6H5NH3+ (aq) + OH− (aq)
Because C6H5NH2 accepts a proton, it is the Brønsted-Lowry base. The H2O molecule, because it donates a proton, is the Brønsted-Lowry acid.
✏️ Exercise \(\PageIndex{1}\)
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
H3PO4 + H2O ⇌ H3O+ + H2PO4−
- Answer
- Brønsted-Lowry acid: H3PO4; Brønsted-Lowry base: H2O
Conjugate Acid-Base Pair
In reality, all acid-base reactions involve the transfer of protons between acids and bases. Let's consider again the acid-base reaction that takes place when ammonia is dissolved in water. As previously established, NH3 is a base since it accepts a proton from H2O and H2O is an acid since it donates a proton to the NH3.
What if the reaction were to happen in reverse? In the reverse reaction, the ammonium ion, NH4+, donates a proton (H+) to the hydroxide ion, OH–, yielding a molecule of NH3 and a molecule of H2O. Since NH4+ acts as a proton donor in the reverse reaction, it would be considered an acid. Since OH– acts as a proton acceptor in the reverse reaction, it would be considered a base.
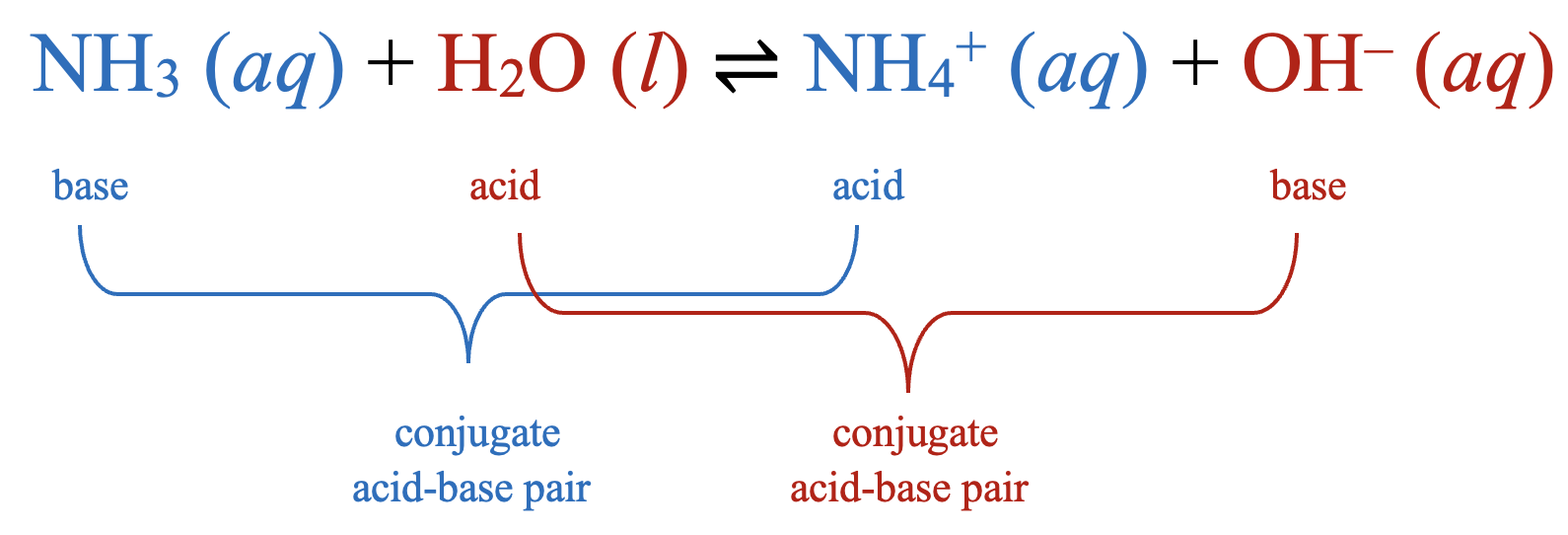
Notice that NH3 and NH4+ differ from each other by one proton (H+). This relationship is called a conjugate acid-base pair or sometimes simply a conjugate pair. Within a conjugate acid-base pair, the species that holds the extra H+ is considered the acid within the pair. This means that within this pair, NH3 is the base and NH4+ is the acid.
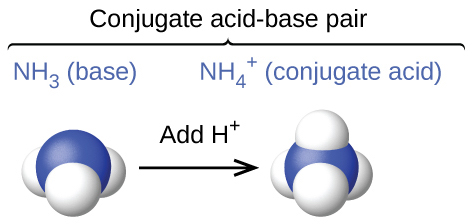
By now you also probably noticed that H2O and OH– differ from each other by one proton (H+). This means they are also a conjugate acid-base pair. Since H2O holds the extra H+ in the pair, H2O is the acid and OH– is the base.
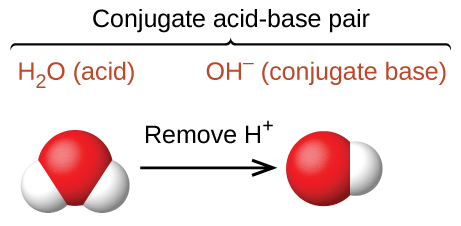
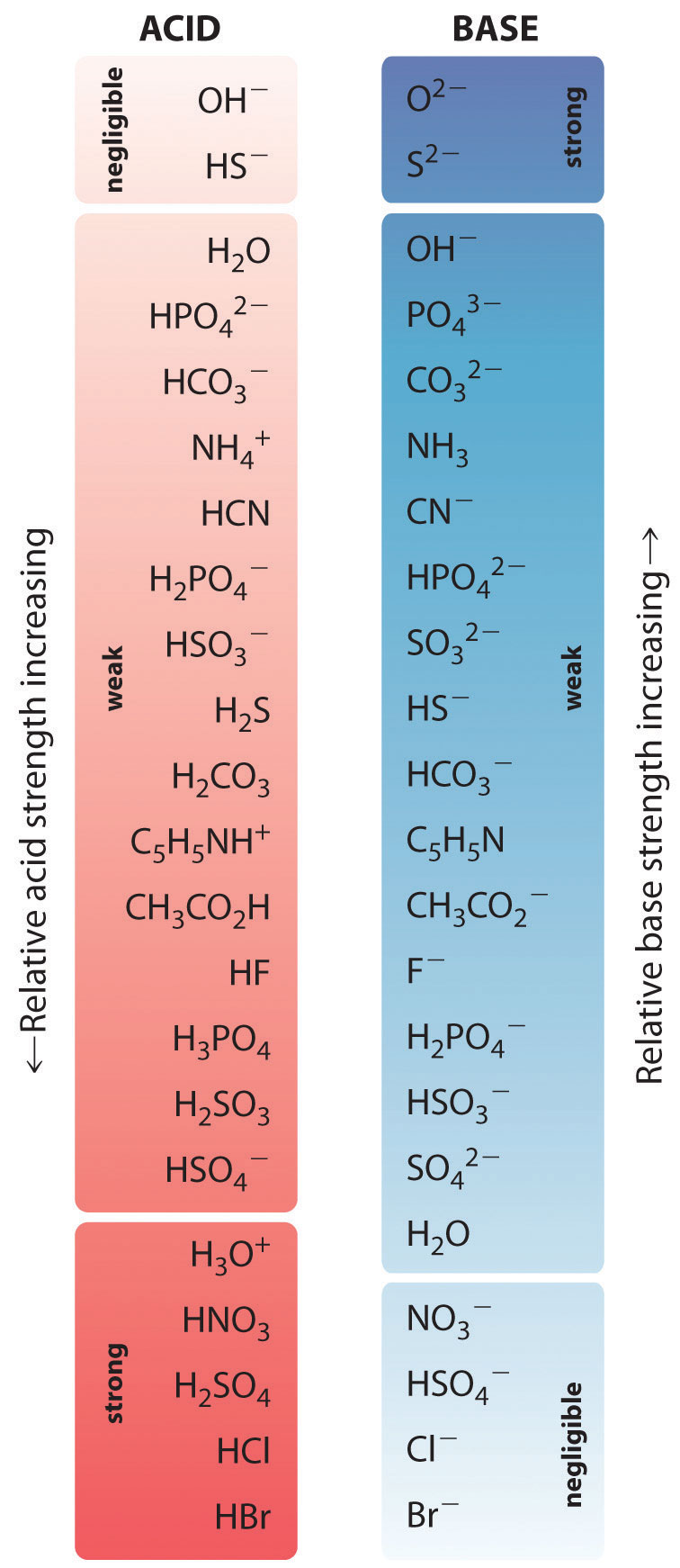
Figure \(\PageIndex{3}\) shows several conjugate acid-base pairs. The acid appears in the first column, while its conjugate base appears in the same row in the second column. Notice again that all conjugate acid-base pairs differ from each other by a single proton (H+).
✅ Example \(\PageIndex{2}\)
Identify the conjugate acid-base pairs in this equilibrium.
HC2H3O2 + H2O ⇌ H3O+ + C2H3O2–
Solution
In this reaction, acetic acid, HC2H3O2, donates a proton to H2O. This means HC2H3O2 is the acid and H2O is the base. In the reverse reaction, H3O+ is the acid since it donates a proton to the acetate ion, C2H3O2–. This means that C2H3O2– is a base.
The two conjugate acid-base pairs:
- HC2H3O2 (acid) and C2H3O2– (base).
- H2O (base) and H3O+ (acid).
✅ Example \(\PageIndex{3}\)
What is the conjugate acid of NO3–?
Solution
The conjugate acid in the pair always has one more proton (H+) than its base. Adding an H+ to NO3– yields the acid HNO3.
✏️ Exercise \(\PageIndex{2}\)
Identify the conjugate acid-base pairs in this reaction.
N2H4 + H2O ⇌ N2H5+ + OH–
- Answer
-
- N2H4 (base) and N2H5+ (acid)
- H2O (acid) and OH– (base)
✏️ Exercise \(\PageIndex{3}\)
What is the conjugate base of H2CO3?
- Answer
- HCO3–
This page is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew, Henry Agnew and Lance S. Lund (Anoka-Ramsey Community College).

