13.9: Exercises
- Page ID
- 357336
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)13.1: Properties of Liquids and Solids
- In terms of their bulk properties, how do liquids and solids differ? How are they similar?
- Answer
-
Liquids and solids are similar in that they are matter composed of atoms, ions, or molecules. They are incompressible and have similar densities that are both much larger than those of gases. They are different in that liquids have no fixed shape, and solids are rigid.
- Explain why liquids assume the shape of any container into which they are poured, whereas solids are rigid and retain their shape.
- Answer
-
Liquids have enough energy to move about relative to each other while remaining in contact. Solids have less energy and remain in a fixed position.
- The density of liquid NH3 is 0.64 g/mL; the density of gaseous NH3 at STP is 0.0007 g/mL. Explain the difference between the densities of these two phases.
- Answer
-
A liquid is a condensed phase where the particles are in constant contact with each other. Gases have enough energy for the particles to separate from each other and spread out. This means that there are fewer gas particles in a given volume than liquid particles.
13.2: Evaporation and Condensation
- Why does spilled gasoline evaporate more rapidly on a hot day than on a cold day?
- Answer
-
As the temperature increases, the average kinetic energy of the molecules of gasoline increases and so a greater fraction of molecules have sufficient energy to escape from the liquid than at lower temperatures.
- How does the boiling of a liquid differ from its evaporation?
- Answer
-
When evaporating, only a few molecules at the surface of the liquid with sufficient energy are able to escape to the gas phase. When boiling, enough molecules in the interior of the liquid have sufficient energy to create small bubbles of gas that are able to move to the surface and escape.
13.3: Melting, Freezing, and Sublimation
- Another way to minimize freezer burn is to wrap food tightly before freezing. Why would this minimize freezer burn?
- Answer
-
Freezer burn is due to the sublimation of solid water to gaseous water. In order to form the gas, there needs to be space for the gas molecules to move about in. Minimizing this space by tightly wrapping the food will minimize the amount of freezer burn possible.
- If a severe storm results in the loss of electricity, it may be necessary to use a clothesline to dry laundry. In many parts of the country in the dead of winter, the clothes will quickly freeze when they are hung on the line. If it does not snow, will they dry anyway? Explain your answer.
- Answer
-
Yes, ice will sublime, although it may take it several days. Ice has a small vapor pressure, and some ice molecules form gas and escape from the ice crystals. As time passes, more and more solid converts to gas until eventually the clothes are dry.
13.4: Energetics of Phase Changes
- Ethyl chloride (boiling point, 13°C) is used as a local anesthetic. When the liquid is sprayed on the skin, it cools the skin enough to freeze and numb it. Explain the cooling effect of liquid ethyl chloride.
- Answer
-
The thermal energy (heat) needed to evaporate the liquid is removed from the skin.
- Heat is added to boiling water. Explain why the temperature of the boiling water does not change. What does change?
- Answer
-
The heat is absorbed by the water to help the molecules overcome the intermolecular attractive forces in the liquid to allow the gas to form. The water remains at 100°C until all of the water has boiled off. Only the volume of the liquid water will change as it is boiling. Once all has been converted to the gas phase, the temperature of the gas will rise.
- Heat is added to ice at 0°C. Explain why the temperature of the ice does not change. What does change?
- Answer
-
The heat is absorbed by the ice, providing the energy required to partially overcome intermolecular attractive forces in the solid and causing a phase transition to liquid water. The solution remains at 0 °C until all the ice is melted. Only the amount of water existing as ice changes until the ice disappears. Then the temperature of the water can rise.
13.5: Electronegativity and Polarity
- Explain the difference between a nonpolar covalent bond, a polar covalent bond, and an ionic bond.
- Answer
-
A nonpolar bond is one where the electrons are shared relatively equally between the two atoms involved in the bond. This will be the case when their electronegativities are very similar (less than 0.4). A polar bond is one where the electrons are shared, but the sharing is not equal causing the more electronegative atoms to have a partial negative charge and the less electronegative atom to have a partial positive charge. This will be the case when the electronegativities differ by 0.4 to about 1.9. Ionic bonds are formed when one atom is able to take the electron totally away from another, forming ions. This will be the case when metals and nonmetals are combined where the difference in electronegativities become 2.0 or more.
- Give an example of a nonpolar covalent bond. How do you know it is nonpolar?
- Answer
-
C—C; The two atoms in the bond are identical so they will share the electrons equally.
- Give an example of a polar covalent bond. How do you know it is polar?
- Answer
-
C—F; The two atoms in the bond have different electronegativities. They are both nonmetals so the difference is likely to be less than 2.0. Fluorine is more electronegative than carbon so the fluorine will have the partial negative charge.
- From its position in the periodic table, determine which atom in each pair is more electronegative:
- Br or Cl
- N or O
- S or O
- P or S
- Si or N
- Ba or P
- N or K
- Answer
-
- Cl
- O
- O
- S
- N
- P
- N
- From its position in the periodic table, determine which atom in each pair is more electronegative:
- N or P
- N or Ge
- S or F
- Cl or S
- H or C
- Se or P
- C or Si
- Answer
-
- N
- N
- F
- Cl
- C
- Se
- C
- Identify the more polar bond in each of the following pairs of bonds:
- H—F or H—Cl
- N—O or C—O
- S—H or O—H
- P—Cl or S—Cl
- C—H or N—H
- S—O or P—O
- C—N or N—N
- Answer
-
- H—F
- C—O
- O—H
- P—Cl
- N—H
- P—O
- C—N
- Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?
- Answer
-
N, O, F, and Cl
- Which is the most polar bond?
- C—C
- C—H
- N—H
- O—H
- Se—H
- Answer
-
d. O—H
- Which bond do you expect to be more polar: an O—H bond or an N—H bond? Why?
- Answer
-
O—H because there is a larger difference in electronegativities
- Which bond do you expect to be more polar: an O—F bond or an S—O bond? Why?
- Answer
-
S—O because there is a larger difference in electronegativities
- How do you know which side of a polar bond has the partial negative charge? Identify the negatively charged side of each polar bond.
- H—Cl
- H—S
- Answer
-
The side that has the more electronegative atom has the partial negative charge.
- Cl
- S
- How do you know which side of a polar bond has the partial positive charge? Identify the positively charged side of each polar bond.
- H—Cl
- N—F
- Answer
-
The side that has the less electronegative atom has the partial positive charge.
- H
- N
- Label the bond between the given atoms as nonpolar covalent, polar covalent, or ionic.
- H and C
- C and F
- K and F
- Answer
-
- nonpolar covalent
- polar covalent
- ionic
- Label the bond between the given atoms as nonpolar covalent, polar covalent, or ionic.
- S and Cl
- P and O
- Cs and O
- Answer
-
- polar covalent
- polar covalent
- ionic
- From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
- C, F, H, O, N
- H, Br, I, F, Cl
- S, P, F, O, H
- Al, P, O, N, Na
- Answer
-
- H < C < N < O < F
- H < I < Br < Cl < F
- H < P < S < O < F
- Na < Al < P < N < O
- Explain how a molecule that contains polar bonds can be nonpolar.
- Answer
-
As long as the polar bonds are opposite of each other and can "cancel" each other out, the molecule can be nonpolar.
- Identify the molecules with a dipole moment:
- CF4
- Cl2CCBr2 (the two C atoms are central)
- CH3Cl
- H2CO
- Answer
-
Cl2CCBr2, CH3Cl, H2CO
- Which of the following molecules have dipole moments?
- CS2
- SeS2
- CCl2F2
- PCl3
- ClNO (N is the central atom)
- Answer
-
SeS2, CCl2F2, PCl3, ClNO
13.7: Intermolecular Forces
- Define the following and give an example of each:
- dispersion force
- dipole-dipole attraction
- hydrogen bond
- Answer
-
A dispersion is the attraction between a partial positive and a partial negative region on neighboring molecules that is caused by the random movement of electrons. This would be the attractive force in hydrocarbons like butane. A dipole-dipole attraction is also the attraction between a partial positive and a partial negative region on neighboring polar molecules. However, in this case it is due to the presence of a permanent dipole in the molecules and is therefore typically stronger than a dispersion force. An example of this would be the attractive force in acetone. A hydrogen bond is a special type of dipole-dipole attraction with the highly polar bond by formed by H—N, H—O, or H—F. This is the seen in water.
- All other things being equal, rank the intermolecular forces in order of increasing strength.
- Answer
-
dispersion force < dipole-dipole attraction < hydrogen bond
- Which subatomic particles (protons, neutrons, electrons) are most responsible for intermolecular forces? Explain your answer.
- Answer
-
electrons; intermolecular forces are caused by partial charges on atoms due to the unequal distribution of electrons in bonds whether that be due to permanent dipoles in polar molecules or temporary dipoles in nonpolar molecules
- Can a molecule experience more than one intermolecular force at the same time? Why or why not?
- Answer
-
Yes. All molecules have dispersion forces. In addition, polar molecules can have dipole-dipole attractions and sometimes hydrogen bonding.
- Of the properties boiling point, structure of the solid phase, and molar mass, which are influenced by hydrogen bonding? Explain your answer.
- Answer
-
Boiling point and the structure of the solid phase are influenced by hydrogen bonding. Both of these are determined by how molecules interact with each other, which can be influenced by hydrogen bonding. Molar mass is simply the mass of the atoms in a molecule and is not affected by how they interact with each other.
- What is the evidence that all neutral atoms and molecules exert attractive forces on each other?
- Answer
-
All atoms and molecules will condense into a liquid or solid in which the attractive forces exceed the kinetic energy of the molecules, at sufficiently low temperature.
- What is the relationship between the intermolecular forces in a solid and its melting temperature?
- Answer
-
As the strength of the intermolecular forces increases, the melting point (or freezing point) will also increase.
- The molecular mass of butanol, C4H9OH, is 74.14; that of ethylene glycol, CH2(OH)CH2OH, is 62.08, yet their boiling points are 117.2 °C and 174 °C, respectively. Explain the reason for the difference.
- Answer
-
Ethylene glycol has more -OH groups than butanol so it is able to form more hydrogen bonding interactions with its neighbors, increasing the boiling point.
- On the basis of intermolecular attractions, explain the differences in the boiling points of n–butane (−1 °C) and chloroethane (12 °C), which have similar molar masses.
- Answer
-
Only rather small dispersion forces from C-H bonds are available to hold n-butane in the liquid state. Chloroethane, however, has rather large dipole interactions because of the Cl-C bond; the interaction is therefore stronger, leading to a higher boiling point.
- On the basis of dipole moments and/or hydrogen bonding, explain in a qualitative way the differences in the boiling points of acetone (56.2 °C) and 1-propanol (97.4 °C), which have similar molar masses.
- Answer
-
While both are polar molecules, 1-propanol is an alcohol so it has an -OH group in the molecule. This -OH group can participate in hydrogen bonding which will increase the strength of the intermolecular forces in the molecules and increase the boiling point.
- The melting point of H2O(s) is 0 °C. Would you expect the melting point of H2S(s) to be −85 °C, 0 °C, or 185 °C? Explain your answer.
- Answer
-
−85 °C. Water has stronger hydrogen bonds so it melts at a higher temperature.
- Silane (SiH4), phosphine (PH3), and hydrogen sulfide (H2S) melt at −185 °C, −133 °C, and −85 °C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?
- Answer
-
All three compounds have similar molar masses, so the difference in melting points is due to just the polar character. Silane with the lowest melting point will have the least polar character and the weakest intermolecular attractions of the three. Hydrogen sulfide with the highest melting point will have the most polar character and the greatest intermolecular attractions of the three.
- Explain why a hydrogen bond between two water molecules is weaker than a hydrogen bond between two hydrogen fluoride molecules.
- Answer
-
The hydrogen bond between two hydrogen fluoride molecules is stronger than that between two water molecules because the electronegativity of F is greater than that of O. Consequently, the partial negative charge on F is greater than that on O. The hydrogen bond between the partially positive H and the larger partially negative F will be stronger than that formed between H and O.
- Under certain conditions, molecules of acetic acid, CH3COOH, form “dimers,” pairs of acetic acid molecules held together by strong intermolecular attractions:
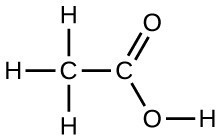
Draw a dimer of acetic acid, showing how two CH3COOH molecules are held together, and stating the type of IMF that is responsible.
- Answer
-
The IMF responsible is hydrogen bonding.
- Arrange each of the following sets of compounds in order of increasing boiling point temperature:
- HCl, H2O, SiH4
- F2, Cl2, Br2
- CH4, C2H6, C3H8
- O2, NO, N2
- Answer
-
- SiH4 < HCl < H2O
- F2 < Cl2 < Br2
- CH4 < C2H6 < C3H8
- N2 < O2 < NO
- Identify the all of the intermolecular forces present in the following solids. Which is the strongest for each?
- CH3CH2OH
- CH3CH2CH3
- CH3CH2Cl
- Answer
-
- dispersion, dipole, hydrogen bonding; hydrogen bonding will be strongest
- dispersion only (and thus the strongest)
- dispersion and dipole; dipole will be strongest
- The types of intermolecular forces in a substance are identical whether it is a solid, a liquid, or a gas. Why then does a substance change phase from a gas to liquid or to a solid?
- Answer
-
The difference in phases is based on how much energy the molecules have relative to the strength of the intermolecular forces. If the energy of the molecules is greater than the strength of the intermolecular forces, the molecules will separate and form a gas. If the energy of the molecules is less than the strength of the intermolecular forces, the molecules will be held together in the liquid or solid phase. The temperature where this occurs will depend on the strength of the intermolecular forces present.
- Why do the boiling points of the noble gases increase in the order He < Ne < Ar < Kr < Xe?
- Answer
-
The London forces typically increase as the number of electrons increase.
- Neon and HF have approximately the same molecular masses. Explain why the boiling points of Neon and HF differ.
- Answer
-
Neon is a nonpolar atom held together by only disperson forces. HF is a polar molecule that can exhibit hydrogen bonding between molecules.
This page was adapted from "Beginning Chemistry (Ball)" by LibreTexts and "Chemistry (OpenStax)" by LibreTexts and is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Vicki MacMurdo (Anoka-Ramsey Community College) and Lance S. Lund (Anoka-Ramsey Community College).


