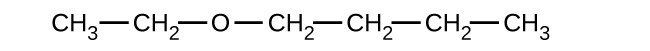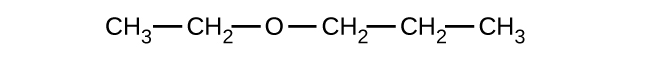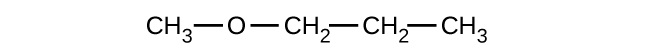12.11: Exercises
- Page ID
- 357335
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)12.4: Branched Alkanes
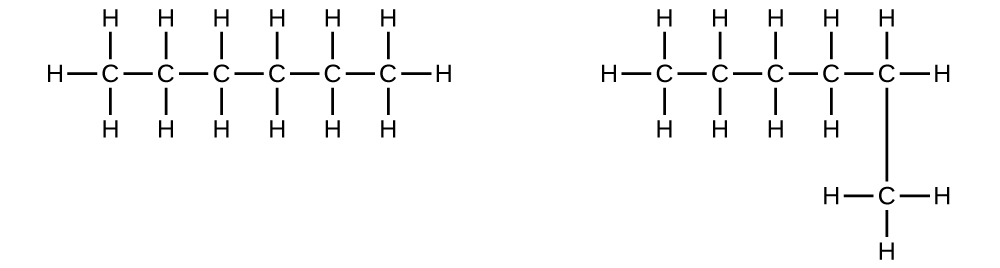
- Explain why these two molecules are not isomers:
- Answer
-
They are the same compound because each is a saturated hydrocarbon containing an unbranched chain of six carbon atoms.
-
Explain why these two molecules are not isomers:
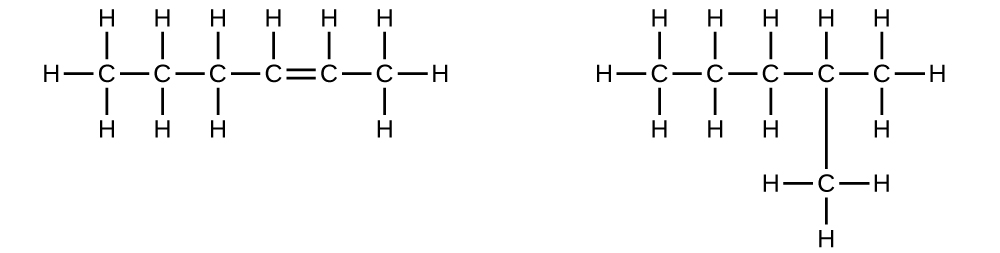
- Answer
-
The molecule on the left has a molecular formula of C6H12 while the one on the right has a molecular formula of C6H14. Since they have different numbers of hydrogens, they cannot be isomers.
- Write the chemical formula and three different Lewis structures for a five-carbon alkane.
- Answer
-
C5H12;
;
;
- What are the IUPAC names of the alkanes drawn in Exercise 3?
- Answer
-
pentane; 2-methylbutane; 2,2-dimethylpropane
- In the gasoline industry, what is called isooctane is actually 2,2,4-trimethylpentane. Draw the condensed structure of isooctane.
- Answer
-
- Isooctane (see Exercise 5) is an isomer of what straight-chain alkane?
- Answer
-
octane
- Name the other three alkane isomers that contain a five-carbon chain with three methyl substituents.
- Answer
-
2,2,3-trimethylpentane; 2,3,4-trimethylpentane; 2,3,3-trimethylpentane
- Draw the Lewis structure and line structure for each of the following hydrocarbons:
- hexane
- 3-methylpentane
- heptane
- 3-ethylhexane
- Answer
-
;
;
;
;
- Give the complete IUPAC name for each of the following compounds:
- CH3CH2CH2CH3
- CH3CH2CH3
- Answer
-
- butane
- 2-methylpropane
- 2-methylbutane
- propane
- 3-methylpentane
- Butane is used as a fuel in disposable lighters. Write the Lewis structure for each isomer of butane.
- Answer
-
;
- Draw line structures of and name the five structural isomers of hexane.
- Answer
-
hexane,
2-methylpentane,
3-methylpentane,
2,3-dimethylbutane,
2,2-dimethylbutane,
12.5: Alkenes and Alkynes
- Draw and name all possible normal (that is, straight-chain) isomers of heptyne.
- Answer
-
1-heptyne,
2-heptyne,
3-heptyne,
- Write the chemical formula and a Lewis structure of the following, each of which contains five carbon atoms:
- an alkene
- an alkyne
- Answer
-
- C5H10;
- C5H8;
- C5H10;
- Explain why unbranched alkenes can form geometric isomers while unbranched alkanes cannot.
- Answer
-
It is very difficult to rotate round a carbon-carbon double bond in an alkene so the location of the groups coming off of it is fixed. There is free rotation about a carbon-carbon single bond in an alkane so the groups coming off of the bond occupy all positions.
- Write the chemical formula, condensed formula, and Lewis structure for each of the following hydrocarbons:
- cis-3-hexene
- 1-pentene
- 3-hexyne
- 2-pentyne
- trans-3-heptene
- 1-hexene
- 2-heptyne
- Answer
-
- C6H12;
;
- C5H10;
;
- C6H10;
;
- C5H8;
;
- C7H14;
;
- C6H12;
;
- C7H12;
;
- C6H12;
- Give the complete IUPAC name for each of the following compounds:
- CH3CH2CH2CH=CH2
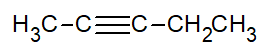
- Answer
-
- 1-butyne
- 1-pentene
- 1-octyne
- trans-2-pentene
- 2-pentyne
- Write Lewis structures for the cis–trans isomers of CH3CH=CHCH3.
- Answer
-
cis:
trans:
- Write Lewis structures and IUPAC names for the alkyne isomers of C4H6.
- Answer
-
1-butyne:
2-butyne:
12.6: Oxygen-Containing Organic Compounds
- Write the condensed structures of both isomers with the formula C2H6O. Label the functional group of each isomer.
- Answer
-
CH3CH2OH, alcohol group; CH3OCH3, ether
- Write the condensed structures of all isomers with the formula C2H6O2. Label the functional group (or groups) of each isomer.
- Answer
-
HOCH2CH2OH, two alcohol groups; CH3OCH2OH, ether and alcohol groups
12.7: Alcohols, Aldehydes, Carboxylic Acids, and Ketones
- Draw the line structures of all the possible isomers of butanol. Include branched isomers.
- Answer
-
,
,
,
- Why do the compounds hexane, hexanol, and hexene have such similar names?
- Answer
-
They all have six carbon chains.
- Write condensed formulas and provide IUPAC names for the following compounds:
- ethyl alcohol (in beverages)
- methyl alcohol (used as a solvent, for example, in shellac)
- Answer
-
ethanol;
methanol;
- Give the complete IUPAC name for each of the following compounds:
- Answer
-
- 2-pentanone
- butanal
- 3-hexanol
- pentanoic acid
- Explain why it is not possible to prepare a ketone that contains only two carbon atoms.
- Answer
-
A ketone contains a group bonded to two additional carbon atoms; thus, a minimum of three carbon atoms are needed.
- Fatty acids are carboxylic acids that have long hydrocarbon chains attached to a carboxylate group. How does a saturated fatty acid differ from an unsaturated fatty acid? How are they similar?
- Answer
-
Since they are both carboxylic acids, they each contain the –COOH functional group and its characteristics. The difference is the hydrocarbon chain in a saturated fatty acid contains no double or triple bonds, whereas the hydrocarbon chain in an unsaturated fatty acid contains one or more multiple bonds.
- Order the following molecules from least to most oxidized, based on the marked carbon atom:
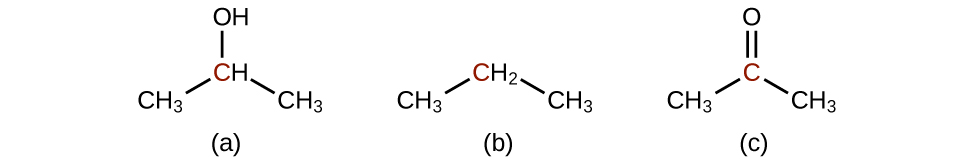
- Answer
-
Most oxidized is c; intermediate is a; least oxidized is b.
- Predict the products of oxidizing the molecules shown in this problem. In each case, identify the product that will result from the minimal increase in oxidation state for the highlighted carbon atom:
- Answer
-
- Predict the products of reducing the following molecules. In each case, identify the product that will result from the minimal decrease in oxidation state for the highlighted carbon atom:
- Answer
-
- Alcohols A, B, and C all have the composition C4H10O. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidized to a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.
- Answer
-
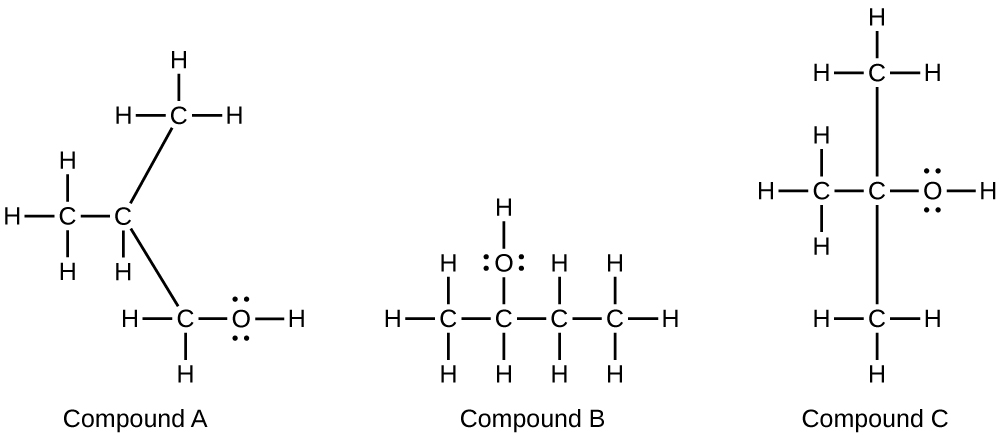
12.8: Esters
- The foul odor of rancid butter is caused by butanoic acid, CH3CH2CH2CO2H, commonly known as butyric acid.
- Draw the Lewis structure of butyric acid.
- The esters formed from butyric acid are pleasant-smelling compounds found in fruits and used in perfumes. Draw the Lewis structure for the ester formed from the reaction of butyric acid with 1-propanol.
- Answer
-
- Write balanced equations for each of the following reactions using condensed formulas:
- ethanol reacts with propanoic acid
- 1-butanol reacts with acetic acid (ethanoic acid)
- Answer
-
12.9: Ethers
- Draw the condensed structure of diethyl ether, once used as an anesthetic.
- Answer
-
CH3CH2OCH2CH3
- Draw the condensed formulas for each of the following compounds:
- dipropyl ether
- ethyl methyl ether
- Answer
-
- CH3CH2CH2OCH2CH2CH3
- CH3CH2OCH3
- Give the common name for each of the following compounds:
- Answer
-
- butyl ethyl ether
- ethyl propyl ether
- methyl propyl ether
Additional Exercises
- Give the IUPAC name for each of the following molecules.
- Answer
-
- 4-ethyl-3-methylheptane
- trans-3-hexene
- 2,4-dimethylhexane
- 3-hexanone
- heptanoic acid
- Write a condensed structural formula for each of the following molecules.
- propene
- 1-butanol
- ethyl propyl ether
- cis-2-heptene
- 2,2,3-trimethylhexane
- methanal (commonly known as formaldehyde)
- Answer
-
- CH2=CHCH3
- CH3CH2CH2CH2OH
- CH3CH2OCH2CH2CH3
- Write a condensed structural formula for each of the following molecules.
- 2-propanol
- acetone (2-propanone)
- dimethyl ether
- acetic acid (ethanoic acid)
- 1-hexene
- Answer
-
- CH3OCH3
- CH2=CHCH2CH2CH2CH3
This page was adapted from "Beginning Chemistry (Ball)" by LibreTexts and "Chemistry (OpenStax)" by LibreTexts and is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Vicki MacMurdo (Anoka-Ramsey Community College) and Lance S. Lund (Anoka-Ramsey Community College).