10.3: Emission Spectra
- Page ID
- 354397
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The incandescent light bulbs that have been phasing out over the past decade or so contain a very thin wire filament that emits light when heated. The filament in these bulbs is typically made of tungsten, since it can be heated to high temperatures without melting. The light emitted by a hot tungsten filament contains virtually every frequency and therefore, appears white. Such a spectrum of frequencies is called a continuous spectrum (no gaps or lines stand out). Every element emits light when energized by heating or passing electric current through it. Solid elements begin to glow when they are heated sufficiently, while elements in gaseous form emit light when electricity passes through them or when they are atomized in a flame. This is the source of light emitted by neon signs and is also the source of light in a fire.
Each Element Has a Unique Spectrum
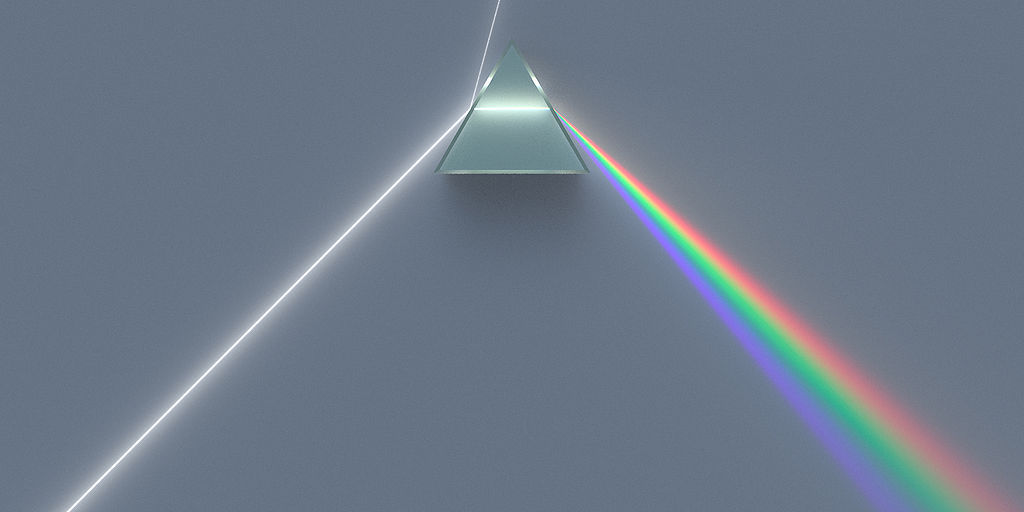
The light frequencies emitted by atoms are mixed together by our eyes so that we see a blended color. Several physicists, including Angstrom in 1868 and Balmer in 1875, passed the light from energized atoms through glass prisms in such a way that the light was spread out so they could see the individual frequencies that made up the light (see Figure \(\PageIndex{1}\)).
The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. However, when separated using a prism or diffraction grating, the hydrogen spectrum reveals four individual frequencies (see top image in Figure \(\PageIndex{2}\)). The pink color of the tube is the result of our eyes blending the four colors.
Every atom has its own characteristic spectrum; no two emission spectra (spectra is the plural form of spectrum) are alike. The images in Figure \(\PageIndex{2}\) above show the emission spectra of hydrogen, helium, magnesium, and xenon, respectively. Video \(\PageIndex{1}\) shows how line spectra may be produced and observed. Since each element has a unique emission spectrum, they are often referred to as the "fingerprints" of the elements.
Applications of Emission Spectra
You may have heard about scientists discussing which elements are present in the sun or a more distant star, and after hearing that, wondered how we could know what elements were present in a place no one has ever been. The elements present in distant stars may be determined by analyzing the spectrum that comes from the stars and observing that there are lines that represent wavelengths that have been absorbed by star's stellar atmosphere. If the exact four lines that compose hydrogen's emission spectrum are present in the lines that had been absorbed by the star's atmosphere then the star contains hydrogen.
While there is some variation in the composition of stars, hydrogen is by far the most abundant element found in stars, followed by helium, and these two elements are found in virtually all stars. Knowing this to be true, it has also been shown that missing lines in the spectra of stars in distant galaxies are not quite identical to the spectra for hydrogen and helium found here on Earth. Instead, they are almost always shifted more toward the reddish portion of the spectrum. It turns out that the more distant the galaxy, the more redshifted the spectra tend to be. Shifts in the spectra are caused by distant galaxies moving away from the Earth. The greater the redshift appears, the greater the speed at which the distant galaxy is moving away from the Earth. This is how scientists know the universe is expanding. See Video \(\PageIndex{2}\) for a short animation.
Additional applications of emission spectra or line spectra include different color "neon" lights, not all of which contain the element neon. Fireworks of different colors are also the result of using different elements, since each element has its own unique spectrum. Video \(\PageIndex{3}\) shows different color flames that are the result of using salts that contain different elements.
Summary
- Each element has its own unique emission spectrum.
This page is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew, Lance S. Lund (Anoka-Ramsey Community College), and Henry Agnew.