12.2: Vapor Pressure
- Page ID
- 435221
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the relationship between atmospheric pressure, vapor pressure and the boiling point of a liquid.
- Understand the principles behind the workings of an autoclave.
Vapor Pressure
When a partially filled container of liquid is sealed with a stopper, some liquid molecules at the surface evaporate into the vapor phase. However, the vapor molecules cannot escape from the container. So, after a certain amount of time, the space above the liquid reaches a point where it cannot hold any more vapor molecules. Now, some of the vapor molecules condense back into a liquid. The system reaches the point where the rate of evaporation is equal to the rate of condensation (see figure 12.2.1). This is considered an equilibrium between the liquid and vapor phase.
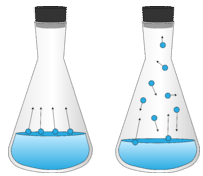
Figure \(\PageIndex{1}\): Equilibrium between liquid phase and vapor phase.
An equilibrium can be illustrated by an equation with a double arrow, meaning that the process is occurring in both directions and at the same rate.
H2O(l)⇌H2O(g)
The forward direction represents the evaporation of water, while the reverse direction represents the condensation of water vapor.
Because the vapor molecules cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The vapor pressure (Pvap) is a measure of the pressure (force per unit area) exerted by a gas above a liquid in a sealed container. Vapor pressure is a property of a liquid based on the strength of its intermolecular forces. A liquid with weak intermolecular forces evaporates more easily and has a higher vapor pressure. A liquid with stronger intermolecular forces does not evaporate easily, and thus has a lower vapor pressure.
Vapor Pressure and Temperature
The temperature of water also plays a role in the magnitude of vapor pressure (Pvap). The vapor pressure of liquids increases with temperature. This is due to increased kinetic energy of molecules at higher temperature. More molecules have the minimum kinetic energy to be bounced of the surface of water. Table 12.2.1 shows the variations of vapor pressure as temperature increases.
Table \(\PageIndex{1}\): Vapor Pressure of Water at Various Temperatures
| Temperature | Vapor Pressure |
| 20.0 oC | 17.5 mm Hg |
| 40.0 oC | 55.3 mm Hg |
| 60.0 oC | 149.4 mm Hg |
| 80.0 oC | 355.1 mm Hg |
| 100.0 oC | 760.0 mm Hg |
| 125.0 oC | 1740.9 mm Hg |
Boiling and Boiling Point
Boiling is a special form of evaporation where conversion from a liquid state to the gaseous state occurs in a liquid through bubble formation. For liquids in open containers the vapor pressure (Pvap) steadily increases as the liquid is heated. The Pvap reaches a value that is equal to the atmospheric pressure (Patm). When these two pressures are equal the vapor bubbles quickly rise to the surface because they are less dense than the liquid itself and the liquid is said to be boiling. The normal boiling point of water is 100.0 oC under an atmospheric pressure of 760.0 mm of Hg (1.00 atm).
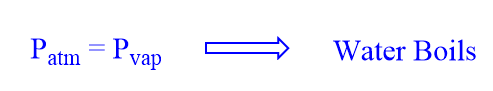
The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the atmospheric pressure.
Altitude and Boiling Point
The actual boiling point depends on the atmospheric pressure. At a pressure greater than 1.00 atm, water boils at a temperature greater than 100.0 °C because the increased pressure forces vapor molecules above the surface to condense. Hence the molecules must have greater kinetic energy to escape from the surface. Conversely, at pressures less than 1.00 atm, water boils below 100.0°C.
At high altitudes, the dependence of the boiling point of water on pressure is significant. Table 12.2.2 lists the boiling points of water at several locations with different altitudes. At an elevation of only 5000 ft, for example, the boiling point of water is already lower than the lowest ever recorded at sea level. The lower boiling point of water has major consequences for cooking. It will take longer to cook a pot of noodles in the Rockies and even longer in the Himalayas. Cake mixes are often sold with separate high-altitude instructions.
| Place | Altitude above Sea Level (ft) | Atmospheric Pressure (mmHg) | Boiling Point of Water (°C) |
|---|---|---|---|
| Mt. Everest, Nepal/Tibet | 29,028 | 240 | 70 |
| Bogota, Colombia | 11,490 | 495 | 88 |
| Denver, Colorado | 5280 | 633 | 95 |
| Washington, DC | 25 | 759 | 100 |
| Dead Sea, Israel/Jordan | −1312 | 799 | 101.4 |
Pressure Cookers and Autoclave
Pressure cookers, which have a seal that allows the pressure inside them to exceed 1 atm, are used to cook food more rapidly by raising the boiling point of water and thus the temperature at which the food is being cooked. At twice the atmospheric pressure of 1520 torr, water boils at 120.0 oC. The high pressures reached in autoclaves, allows water to boil at temperatures high enough to kill most infectious agents. Therefore the autoclave such as in figure 12.2.2 is used for sterilization of surgical equipment in a medical facility and media and equipment in a microbiology laboratory.

Figure \(\PageIndex{2}\): Autoclave: Large autoclave used for moist sterilization of media and equipment.
Summary
The vapor pressure is a measure of the pressure exerted by a gas above a liquid in a closed container. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the atmospheric pressure. Autoclaves, allows water to boil at high temperatures under high pressures. The high temperatures kill most infectious agents.


