11.8: Transport Across Cell Membrane
- Page ID
- 434664
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how various compounds cross the membrane.
- Relate to electron transport chain as examples of facilitated diffusion and active transport of H+ ions.
What is a cell membrane?
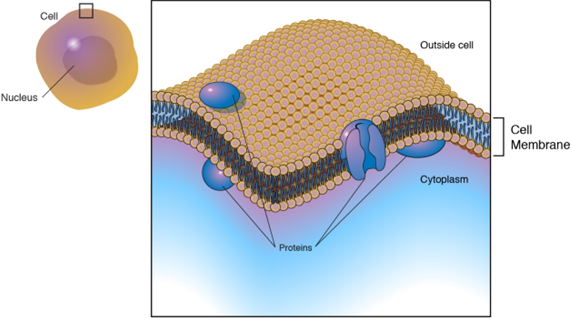 The cell membrane, also known as the plasma membrane is a lipid bilayer that separates the cell interior from the extracellular space.
The cell membrane, also known as the plasma membrane is a lipid bilayer that separates the cell interior from the extracellular space.
Composition of the cell membrane
A cell membrane is a complex structure with several components as shown in Figure \(\PageIndex{1}\). and described here.
- Phospholipid bilayer that has polar heads on the outside in contact with water and nonpolar tails inside the bilayer. Unsaturated fatty acids in the lipid hydrophobic tails increase the membrane fluidity. The more the proportion of unsaturated fatty acids the higher the fluidity.
- Cholesterol interspersed between phospholipids gives rigidity to the membrane. The more the proportion of cholesterol the more rigid the membrane.
- Two types of proteins: integral proteins that span the membrane and serve as transporters of species and peripheral proteins that are loosely attached to the outer side of the membrane that act as enzymes to facilitate the interaction with the cell's environment.
- Glycoproteins and glycolipids have carbohydrate parts attached to the outer lipid layer and serve the purpose of cell-to-cell recognition.
The cell membrane controls the movement of substances in and out of the cells and organelles. It is selectively permeable to ions and organic molecules. It plays a role in cell adhesion, cell signaling, and attachment surface for the cytoskeleton to give the shape to the cells and attached to the extracellular matrix to hold the cells together in tissues.

Transport through the cell membrane
The cell membrane is a partition between intracellular and extracellular spaces, but some substances needed by the cell need to enter and some products or wastes need to exit the cell. The cell membrane allows a selective movement of substances in and out of the cell in several ways.
Diffusion
Nonpolar molecules, such as \(\ce{O2}\), \(\ce{CO2}\), and N2, can move across the membrane from a higher concentration region to a lower concentration region through the process of diffusion, as illustrated in Figure \(\PageIndex{2}\). Diffusion does not require energy and hence this is called passive transport.

Figure \(\PageIndex{2}\): Illustration of diffusion across the cell membrane.
Facilitated Diffusion
Proteins that span the membrane form channels through which polar molecules and ions can diffuse more rapidly than by simple diffusion. The proteins have the channel size that match the size of the substance or they change the shape to adjust to the size of the substance that needs to be selectively transported through the facilitated transport, as illustrated in Figure \(\PageIndex{3}\) right. Ions such as chloride ion (\(\ce{Cl^-}\)), bicarbonate ion (HCO3-), and polar molecules like glucose molecules do not move fast enough through simple diffusion and are transported by the facilitated diffusion process to meet the need of the cells. Movement of water involves facilitated diffusion with the help of protein channels called aquaporins. Movement of water across cell membranes gives rise to osmosis. Facilitated diffusion does not require energy and hence this is also an example of passive transport.

Figure \(\PageIndex{3}\): Illustration of facilitated diffusion transport across the cell membrane.
Active transport
Sometimes substance need to be moved against the concentration gradient, from lower to higher concentration. This process requires the input of energy. Polar molecules and ions are transported across membranes through a protein channel in the direction of lower to higher concentration. For example, \(\ce{K^+}\) concentration is greater inside the cell and \(\ce{Na^+}\) is greater outside the cell. In the conduction of nerve impulses and contraction of muscles, \(\ce{K^+}\) moves into the cell, and \(\ce{Na^+}\) moves out of the cell by active transport. The active transport process, is illustrated in figure 11.8.4.

The Electron Transport Chain
Facilitated diffusion and active transport of the hydrogen ion, (H+) is encountered in a process called the electron transport chain which happens in the inner mitochondrial membrane.
At the end of a turn of the citric acid cycle, energy-rich harvest molecules such as GTP, FADH2 and 3 NADH molecules are produced. The primary task of the last stage of cellular respiration, the electron transport chain, is to transfer the potential energy from NADH and FADH2 to ATP molecules. The ATP molecule is the "battery" which power work within the cell.
The electron transport chain is a group of protein embedded in the inner mitochondrial membrane (see figure 11.8.5). These proteins are energy carrier molecules and are arranged in sequence within the membrane so that energy-carrying electrons pass from one to another, losing a little energy in each step. Complex I accepts electrons from NADH. As a result NADH is oxidized to NAD+. Complex II accepts electrons from FADH2. FADH2 is oxidized to FAD. Electrons supplied by NADH and FADH2 move from one protein complex to another in the inner mitochondrial membrane. The energy is harnessed to pump hydrogen ions, from the matrix (low H+ concentration) into the intermembrane space (high H+ concentration) in a process called active transport. As a result the concentration of concentration of H+ ions become higher in the intermembrane space creating an electrochemical gradient.
Hydrogen ions then flow down the gradient - from high concentration region to low concentration. The ion channel/enzyme ATP synthase allows the H+ to flow from intermembrane space to the matrix in a facilitated diffusion process. The energy released during the facilitated diffusion of hydrogen ions converts a ADP and a phosphate ion to an ATP in a process known as oxidative phosphorylation.

Figure \(\PageIndex{5}\): The electron transport chain embedded in the mitochondrial inner membrane capture high-energy electrons from the carrier molecules and use them to concentrate hydrogen ions in the intermembrane space. Hydrogen ions flow down the gradient back into the matrix through ATP synthase channel which capture the energy to convert ADP to ATP. (CC BY-NC 3.0; Mariana Ruiz Villarreal (LadyofHats) for the CK-12 Foundation).
Oxygen is the final electron acceptor which combines with hydrogen ions to form water. One glucose molecule is converted to approximately 32 ATP's with glycolysis, aerobic conversion of pyruvate to acetyl-CoA, the citric acid cycle, and the electron transport chain. Cellular respiration transfers the energy from one molecule of glucose to molecules of ATP, releasing carbon dioxide and water as waste. Food energy has become energy which can be used for work within the cell such as active transport of molecules across membranes and building large organic and biological molecules.
Summary
There are three different ways molecules and ions move across a cell membrane. They are diffusion, facilitated diffusion, and active transport. Active transport requires energy, while diffusion and facilitated diffusion do not. Most polar molecules and ions require a protein channel during transport.


