5.5: Reactions Involving Water
- Page ID
- 429090
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify the products of hydrolysis of an ester under acidic conditions.
- Describe how to prepare alcohols from alkenes via hydration reaction.
- Describe how to dehydrate an alcohol to an alkene by elimination of a water molecule.
Water is a reactant or product in a number of reactions important to biochemistry. In this section we will take a look at three of them with examples from organic chemistry. They are
- Hydrolysis of esters
- Hydration of alkenes
- Dehydration of alcohols
Hydrolysis
Hydrolysis is, literally “splitting with water.” Hydrolysis is the most important reaction of esters. The hydrolysis of esters is catalyzed by either an acid or a base. The ester is heated with a large excess of water containing a strong-acid catalyst such as sulfuric acid. The sulfuric acid is a source of the hydrogen ion (H+), the catalyst in the reaction.
Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol. Note that the -OH of the carboxylic acid and the H of the alcohol comes from water (shown in purple).

In this next example, butyl acetate and water react to form acetic acid and 1-butanol.
The lysis (splitting) happens at the C-O single bond of the ester indicated with a  .
.
The role of the hydrogen ion (H+) is to increase the speed of the hydrolysis reaction. Therefore H+ is a catalyst in the reaction.

Hydration
Many simple alcohols are made by the hydration of alkenes. Ethanol is made by the hydration of ethylene in the presence of a catalyst such as hydrogen ion (H+). Sulfuric acid (H2SO4) is the source of the hydrogen ion. The alcohol functional group and the hydrogen come from the water molecule (shown in purple). The double bond in the alkene is replaced by a single bond in the alcohol.

In a similar manner, isopropyl alcohol (rubbing alcohol) is produced by the addition of water to propylene. Hydration of an alkene is an example of an addition reaction.

Dehydration
An alcohol undergoes dehydration in the presence of a catalyst to form an alkene and water. The reaction removes the OH group from the alcohol carbon atom and a hydrogen atom from an adjacent carbon atom in the same molecule. Dehydration is the reverse of hydration and is classified as a decomposition reaction.

Reactions With Water Important in Biochemistry
The reaction of organic compounds with water are found in many metabolic processes and happen within the living cells. Here are a few examples from the citric acid cycle, fatty acid catabolism, and glucose metabolism.
a. The citric acid cycle is a series of reactions important to metabolism. In step 7 of the cycle the alkene fumarate undergoes hydration to malate an alcohol.
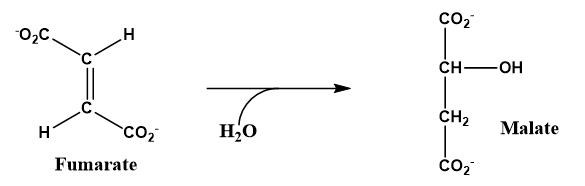
b. In step 2 of the spiral of fatty acid catabolism is a hydration of an alkene to a 2o-alcohol.

c. In step 9 of glycolysis (glucose metabolism) the alcohol 2-phosphoglycerate undergoes dehydration to an alkene.
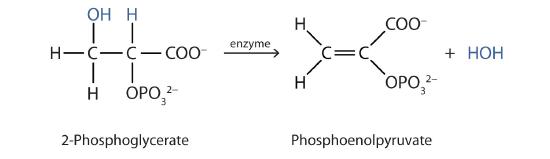
d. Another step in the citric acid cycle involves the dehydration of the citrate ion (3o-alcohol) to an alkene. The alkene then undergoes hydration to form a 2o-alcohol (isocitrate). Citrate and isocitrate are isomers. The subsequent step allows for the oxidation of the 2o-alcohol in isocitrate.

Biochemistry Link
Hydrolysis determines the length of time some drugs remain active. Dental anesthetics like novocain and lidocaine are short acting anesthetics that remain effective for a short time as a pain killer due to hydrolysis reaction. Shown below is the hydrolysis of aspirin which is a pain killer. The ester functional group of aspirin undergoes hydrolysis and forms products salicylic acid and acetic acid. Enzymes are biological catalysts that speed up the hydrolysis reaction.

Although the participating compounds in living systems are complex, the reactions are the same. There is either elimination of water from the starting material, or addition of a water molecule to a starting material, or hydrolysis with water. The idea is that if you know the chemistry of a particular functional group, you know the chemistry of hundreds of different compounds that contain the particular functional group.
This page was constructed from the following sources by Deboleena Roy (American River College):


