4.9: End of Chapter Problems
- Page ID
- 436844
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Formulae of Compounds
1. Write the molecular formula for the following compounds shown in their line bond structure.
a. 
b. 
c. 
2. Draw an isomer of the following compounds.
a. 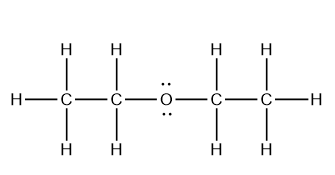
b. 
3. Draw condensed formula for the following compounds shown in their line bond structure.
a. 
b. 
c. 
4. Draw skeletal formula for the compounds in question 3 shown in their line bond structure.
5. The molecules shown below are in their condensed structural formula. Draw line bond formula, molecular formula, and skeletal formula of the compounds.
a. CH3CH2NH2
b. CH3OCH2CH2CH3
c. CH3CH2CH2Cl
d. CH3CH2CH2CH2CH3
Shapes of Molecules
What is the geometry around the central atom?
a. N of the ammonium ion, NH4+
b. N of ammonia, NH3
c. C of methane, CH4
d. O of water, H2O
Bond Polarity, and Overall Polarity
1. Describe the electronegativity difference between each pair of atoms and the resulting polarity (or bond type).
a. C and H
b. H and H
c. Na and Cl
d. O and H
2. Describe the electronegativity (EN) difference between each pair of atoms and the resulting polarity (or bond type).
a. C and O
b. K and Br
c. N and N
d. Cs and F
3. Which of the following molecules are polar? (Hint: Draw the molecules in their line bond formula around the central atom carbon)
a. CH3F
b. CH2F2
c. CF4
4. Which of the following molecules are polar? (Hint: Draw the molecules in their line bond formula and label the polar bonds.)
a. CH3OH
b. CH3COCH3
c. CH3CH2CH3
d. CH3NH2
Intermolecular Forces
1. Predict which will have the higher boiling point: N2 or CO. Explain your reasoning.
2. Predict which will have the higher boiling point: ICl or Br2. Explain your reasoning.
3. Consider the compounds dimethylether (CH3OCH3), ethanol (CH3CH2OH), and propane (CH3CH2CH3). Their boiling points are −42.1 °C, −24.8 °C, and 78.4 °C. Match each compound with its boiling point. Explain your reasoning.
4. Ethane (CH3CH3) has a melting point of −183 °C and a boiling point of −89 °C. Will the melting and boiling points for methylamine (CH3NH2) be higher or lower than ethane. Explain your reasoning.
5. Order the following compounds of group 4A elements and hydrogen from lowest to highest boiling point: CH4, SiH4, GeH4, and SnH4. Explain your reasoning.
6. Order the following hydrocarbons from lowest to highest boiling point: C2H6, C3H8, and C4H10. Explain.
7. What intermolecular forces besides dispersion forces, if any, exist in each substance?
a. potassium chloride (KCl)
b. ethanol (C2H5OH)
c. bromine (Br2)
8. What intermolecular forces besides dispersion forces, if any, exist in each substance?
methylamine (CH3NH2)
calcium sulfate (CaSO4)
carbon monoxide (CO)
9. Which pair of molecules can form a hydrogen bond with one another?
a. CH3CH3 and CH3CH3
b. CH3SH and CH3SH
c. CH3OH and CH3OH
d. CH3CHO and CH3CHO
10. Which of the molecules in question 9 can form hydrogen bonds with water molecules?
Functional Groups or Families of Compounds
Draw the structures of 10 compounds. Each of the 10 compounds should be an example of the functional group below with the correct # of carbon atoms specified.
a. An alkene with 4 carbons
b. An alcohol with 3 carbons
c. A thiol with 2 carbons.
d. A disulfide with 4 carbons.
e. An amine with 3 carbons. Label as primary, secondary, or tertiary.
f. A ketone with 3 carbons.
g. An aldehyde with 3 carbons.
h. A carboxylic acid with 3 carbons.
i. An ester with 3 carbons.
j. An amide with 2 carbons.
Cis-Trans Isomers
Which compounds can exist as cis-trans (geometric) isomers? Draw the cis and trans isomers.
a. ClCH=CHBr
b. CH2=CBrCH3
c. (CH3)2C=CHCH2CH3
d. CH3CH=CHCH2CH3
e. CH2=CHCH2CH2CH3
f. CH3CH=CHCH2CH3
g. CH3CH2CH=CHCH2CH3


