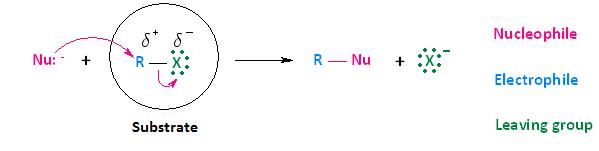
Substrate
- Page ID
- 1151
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Four Factors to Consider in Determining the Relative Ease at Which SN2 Displacement Occurs
- The nature of the leaving group (SN2 Reactions-The Leaving Group)
- The reactivity of the nucleophile (SN2 Reactions-The Nucleophile)
- The solvent (SN2 Reactions-The Nucleophile)
- The structure of the alkyl portion of the substrate (SN2 Reactions-The Substrate)
Sterically Hindered Substrates Will Reduce the SN2 Reaction Rate
Now that we have discussed the effects that the leaving group, nucleophile, and solvent have on biomolecular nucleophilic substitution (SN2) reactions, it's time to turn our attention to how the substrate affects the reaction. Although the substrate, in the case of nucleophilic substitution of haloalkanes, is considered to be the entire molecule circled below, we will be paying particular attention to the alkyl portion of the substrate. In other words, we are most interested in the electrophilic center that bears the leaving group.
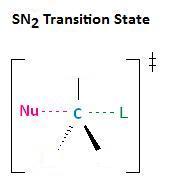
In the section Kinetics of Nucleophilic Substitution Reactions, we learned that the SN2 transition state is very crowded. Recall that there are a total of five groups around the electrophilic center, the nucleophile, the leaving group, and three substituents.
If each of the three substituents in this transition state were small hydrogen atoms, as illustrated in the first example below, there would be little steric repulsion between the incoming nucleophile and the electrophilic center, thereby increasing the ease at which the nucleophilic substitution reaction can occur. Remember, for the SN2 reaction to occur, the nucleophile must be able to attack the electrophilic center, resulting in the expulsion of the leaving group. If one of the hydrogens, however, were replaced with an R group, such as a methyl or ethyl group, there would be an increase in steric repulsion with the incoming nucleophile. If two of the hydrogens were replaced by R groups, there would be an even greater increase in steric repulsion with the incoming nucleophile.

How does steric hindrance affect the rate at which an SN2 reaction will occur? As each hydrogen is replaced by an R group, the rate of reaction is significantly diminished. This is because the addition of one or two R groups shields the backside of the electrophilic carbon, impeding nucleophilic attack.
The diagram below illustrates this concept, showing that electrophilic carbons attached to three hydrogen atoms results in faster nucleophilic substitution reactions, in comparison to primary and secondary haloalkanes, which result in nucleophilic substitution reactions that occur at slower or much slower rates, respectively. Notice that a tertiary haloalkane, that which has three R groups attached, does not undergo nucleophilic substitution reactions at all. The addition of a third R group to this molecule creates a carbon that is entirely blocked.

Substitutes on Neighboring Carbons Slow Nucleophilic Substitution Reactions
Previously we learned that adding R groups to the electrophilic carbon results in nucleophilic substitution reactions that occur at a slower rate. What if R groups are added to neighboring carbons? It turns out that the addition of substitutes on neighboring carbons will slow nucleophilic substitution reactions as well.
In the example below, 2-methyl-1-bromopropane differs from 1-bromopropane in that it has a methyl group attached to the carbon that neighbors the electrophilic carbon. The addition of this methyl group results in a significant decrease in the rate of a nucleophilic substitution reaction.

If R groups were added to carbons farther away from the electrophilic carbon, we would still see a decrease in the reaction rate. However, branching at carbons farther away from the electrophilic carbon would have a much smaller effect.

