CX6. Semi-Anionic Nucleophiles
- Page ID
- 4248
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carboxyloids can be interconverted through addition of typical heteroatomic nucleophiles: amines, alcohols, thiols and water. In addition, other nucleophiles can displace the "leaving group" on a carboxyloid, provided the nucleophile is reactive enough.
Problem CX6.1.
Could a halide, such as bromide or chloride, replace a carboxyloid leaving group easily? Explain.
Carbon and hydrogen anions (or "semianions") are very good nucleophiles. Earlier, we saw how they can react with simple carbonyls. Although the lone pair on a carbon or a hydrogen is often masked in a covalent bond with a moderately electropositive metal such as aluminum or magnesium, that bonding pair of electrons is still nucleophilic enough to donate to a good electrophile. These nucleophiles can often react with carboxylic acid derivatives.
Problem CX6.2.
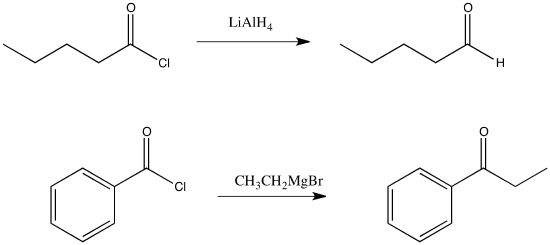
Draw a mechanism for the replacement of the chloride in propanoyl chloride with a hydride from lithium aluminum hydride.
Problem CX6.3.
Draw a mechanism for the replacement of the chloride in propanoyl chloride with a methyl from methylmagnesium chloride.
Addition of a Grignard reagent (alkylmagnesium halide) to a carboxyloid results in the formation of a ketone. Addition of a complex hydride reagent (such as lithium aluminum hydride) to a carboxyloid results in the formation of an aldehyde. We have already seen that these reagents can add to aldehydes and ketones to afford alcohols.
So, what happens if a Grignard reagent is added to an acid chloride or an acid anhydride? The carboxyloid would be converted to a ketone. If there are still more Grignard molecules around, they would probably convert the ketone into an alkoxide ion (and ultimately an alcohol via protonation). The thing is, it is very likely that there will be more Grignard molecules around. A reaction tends to involve millions of reactant molecules, so by the time the first thousand or so molecules of carboxyloid have been converted to ketone, hundreds of those ketone molecules have already been converted to alkoxide.
In most cases, alkyl reagents and hydride reagents will add twice to carboxyloids. They will convert the carboxyloid into an aldehyde or ketone. Because aldehydes and ketones also react with hydride and alkyl nucleophiles, they will react a second time.
Figure CX6.1. A modified potential energy surface that includes aldehydes and ketones.
Problem CX6.4.
Grignard reagents will not effect leaving group replacement in carboxylic acids. Show why that particular reaction does not occur, with the help of a mechanism.
Problem CX6.5.
Grignard reagents generally do not react with either amides or carboxylate ions. Explain why.
When a series of compounds varies from more reactive to less reactive in a particular reaction type, the possibility for selectivity arises. Some reagents may react with a few carboxyloids, but not with others. For example, organocuprates such as (CH3)2CuLi, which do not generally react well with aldehydes and ketones, are very selective in terms of which carboxyloids they will react with.
Problem CX6.6.
Which carboxyloids do you think will react with (CH3)2CuLi? Show the reaction in each case (the reactant, reagent, and a reaction arrow going to the product). Explain your reasoning.
Problem CX6.7.
Complex hydride reagents can be very selective towards carboxyloids. For example, sodium borohydride is not powerful enough to react with esters.
- Which of the carboxyloids can sodium borohydride react with? Explain.
- What other carboxyloids can sodium borohydride NOT react with? Explain.
Problem CX6.8.
Lithium aluminum hydride can induce carboxylic substitution with carboxylate salts such as sodium octanoate.
- What would be the ultimate product of this reaction? Explain.
- What other carboxyloids can lithium aluminum hydride react with? Explain.
Problem CX6.9.
- Which is more reactive: lithium aluminum hydride or sodium borohydride?
- Which is more selective: lithium aluminum hydride or sodium borohydride?
- How can you explain the difference in reactivity between lithium aluminum hydride and sodium borohydride?
Problem CX6.10.
The reaction of lithium aluminum hydride with amides is unusual in that the final product of the reaction is generally an amine.
- Why does this reaction seem to be different from other carboxyloid reactions?
- Draw a mechanism for this reaction. (Hint: at some point, an oxygen atom donates a pair of electrons to aluminum.)
- Propose a reason why this hydride reaction follows a different path than other reactions of hydrides with carboxyloids.