Electromagnetic Radiation
- Page ID
- 1779
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As you read the print off this computer screen now, you are reading pages of fluctuating energy and magnetic fields. Light, electricity, and magnetism are all different forms of electromagnetic radiation.
Introduction
Electromagnetic radiation is a form of energy that is produced by oscillating electric and magnetic disturbance, or by the movement of electrically charged particles traveling through a vacuum or matter. The electric and magnetic fields come at right angles to each other and combined wave moves perpendicular to both magnetic and electric oscillating fields thus the disturbance. Electron radiation is released as photons, which are bundles of light energy that travel at the speed of light as quantized harmonic waves. This energy is then grouped into categories based on its wavelength into the electromagnetic spectrum. These electric and magnetic waves travel perpendicular to each other and have certain characteristics, including amplitude, wavelength, and frequency.
General Properties of all electromagnetic radiation:
- Electromagnetic radiation can travel through empty space. Most other types of waves must travel through some sort of substance. For example, sound waves need either a gas, solid, or liquid to pass through in order to be heard.
- The speed of light is always a constant. (Speed of light : 2.99792458 x 108 m s-1)
- Wavelengths are measured between the distances of either crests or troughs. It is usually characterized by the Greek symbol \(\lambda\).
Waves and their Characteristics
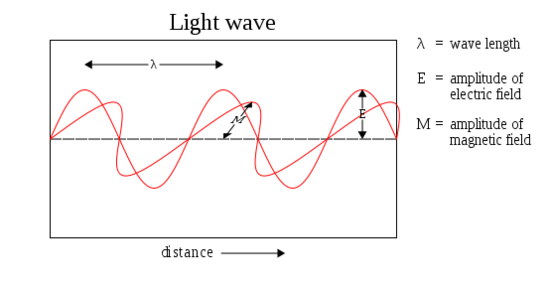
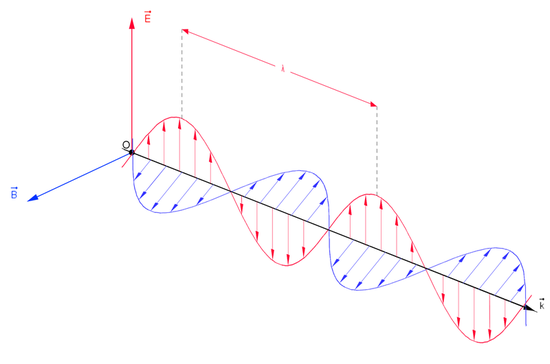
Fig. 1 & 2: Electromagnetic Waves
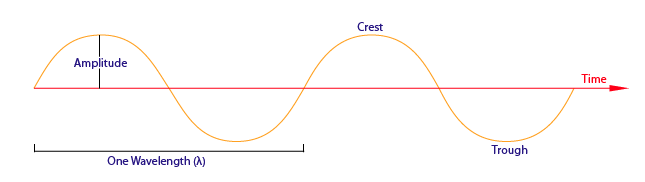
Fig. 3: An EM Wave
Amplitude
Amplitude is the distance from the maximum vertical displacement of the wave to the middle of the wave. This measures the magnitude of oscillation of a particular wave. In short, the amplitude is basically the height of the wave. Larger amplitude means higher energy and lower amplitude means lower energy. Amplitude is important because it tells you the intensity or brightness of a wave in comparison with other waves.
Wavelength
Wavelength (\(\lambda\)) is the distance of one full cycle of the oscillation. Longer wavelength waves such as radio waves carry low energy; this is why we can listen to the radio without any harmful consequences. Shorter wavelength waves such as x-rays carry higher energy that can be hazardous to our health. Consequently lead aprons are worn to protect our bodies from harmful radiation when we undergo x-rays. This wavelength frequently relationship is characterized by:
\[ c = \lambda\nu \]
where
- c is the speed of light,
- \( \lambda \) is wavelength, and
- \( \nu \) is frequency.
Shorter wavelength means greater frequency, and greater frequency means higher energy. Wavelengths are important in that they tell one what type of wave one is dealing with.
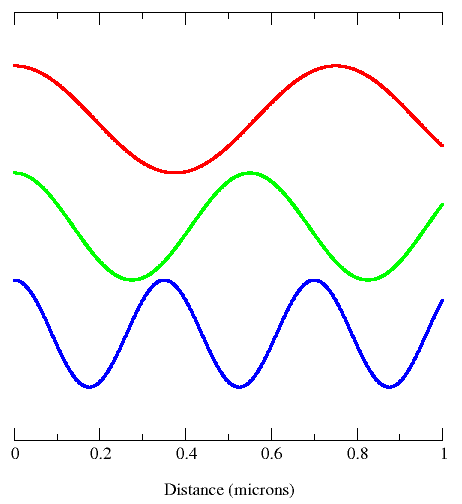
Fig. 4: Different Wavelengths and Frequencies
Remember, Wavelength tells you the type of light and Amplitude tells you about the intensity of the light
Frequency
Frequency is defined as the number of cycles per second, and is expressed as sec-1 or Hertz (Hz). Frequency is directly proportional to energy and can be express as:
\[ E = h\nu \]
where
- E is energy,
- h is Planck's constant, (h= 6.62607 x 10-34 J), and
- \(\nu\) is frequency.
Period
Period (T) is the amount of time a wave takes to travel one wavelength; it is measured in seconds (s).
Velocity
The velocity of wave in general is expressed as:
\[ velocity = \lambda\nu \]
For Electromagnetic wave, the velocity in vacuum is \(2.99 \times 10^8\;m/s\) or \(186,282\) miles/second.
Electromagnetic spectrum
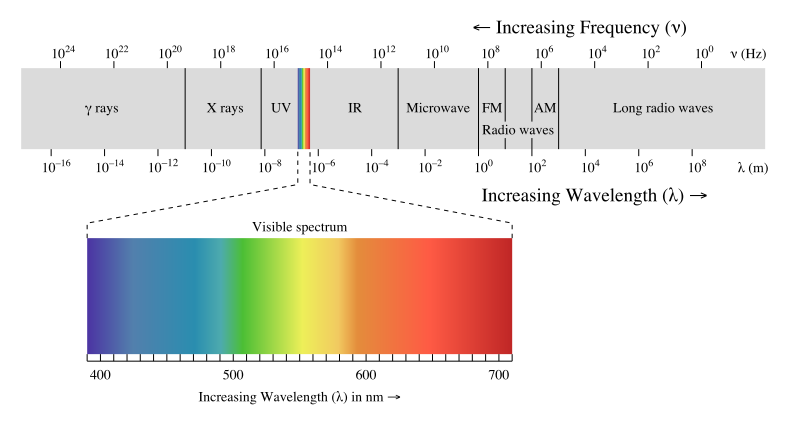
Figure 24.5.1: Electromagnetic spectrum with light highlighted. from Wikipedia.
As a wave’s wavelength increases, the frequency decreases, and as wave’s wavelength decreases, the frequency increases. When electromagnetic energy is released as the energy level increases, the wavelength decreases and frequency decreases. Thus, electromagnetic radiation is then grouped into categories based on its wavelength or frequency into the electromagnetic spectrum. The different types of electromagnetic radiation shown in the electromagnetic spectrum consists of radio waves, microwaves, infrared waves, visible light, ultraviolet radiation, X-rays, and gamma rays. The part of the electromagnetic spectrum that we are able to see is the visible light spectrum.
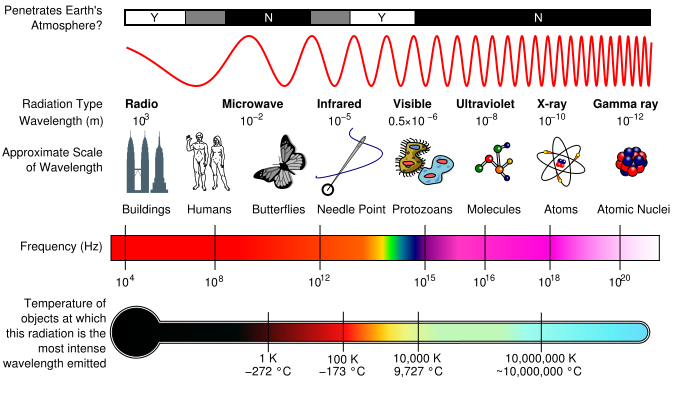
Fig. 6: Electromagnetic Spectrum with Radiation Types
Radiation Types
Radio Waves are approximately 103 m in wavelength. As the name implies, radio waves are transmitted by radio broadcasts, TV broadcasts, and even cell phones. Radio waves have the lowest energy levels. Radio waves are used in remote sensing, where hydrogen gas in space releases radio energy with a low frequency and is collected as radio waves. They are also used in radar systems, where they release radio energy and collect the bounced energy back. Especially useful in weather, radar systems are used to can illustrate maps of the surface of the Earth and predict weather patterns since radio energy easily breaks through the atmosphere. ;

Microwaves can be used to broadcast information through space, as well as warm food. They are also used in remote sensing in which microwaves are released and bounced back to collect information on their reflections.
Microwaves can be measured in centimeters. They are good for transmitting information because the energy can go through substances such as clouds and light rain. Short microwaves are sometimes used in Doppler radars to predict weather forecasts.

Infrared radiation can be released as heat or thermal energy. It can also be bounced back, which is called near infrared because of its similarities with visible light energy. Infrared Radiation is most commonly used in remote sensing as infrared sensors collect thermal energy, providing us with weather conditions.

This picture represents a snap shot in mid-infrared light.
Visible Light is the only part of the electromagnetic spectrum that humans can see with an unaided eye. This part of the spectrum includes a range of different colors that all represent a particular wavelength. Rainbows are formed in this way; light passes through matter in which it is absorbed or reflected based on its wavelength. Thus, some colors are reflected more than other, leading to the creation of a rainbow.
|
Color Region |
Wavelength (nm) |
| Violet | 380-435 |
| Blue | 435-500 |
| Cyan | 500-520 |
| Green | 520-565 |
| Yellow | 565-590 |
| Orange | 590-625 |
| Red | 625-740 |
Fig. 7: The color regions of the Visible Spectrum
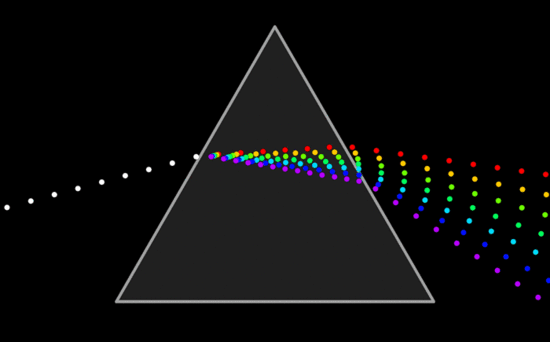
Fig. 8: Dispersion of Light Through A Prism
Ultraviolet, Radiation, X-Rays, and Gamma Rays are all related to events occurring in space. UV radiation is most commonly known because of its severe effects on the skin from the sun, leading to cancer. X-rays are used to produce medical images of the body. Gamma Rays can used in chemotherapy in order to rid of tumors in a body since it has such a high energy level. The shortest waves, Gamma rays, are approximately 10-12 m in wavelength. Out this huge spectrum, the human eyes can only detect waves from 390 nm to 780 nm.
Equations of Waves
The mathematical description of a wave is:
\[ y = A\sin(kx - \omega{t}) \]
where A is the amplitude, k is the wave number, x is the displacement on the x-axis.
\[ k = \dfrac{2\pi}{\lambda} \]
where \(\lambda\) is the wavelength. Angular frequency described as:
\[ \omega = 2\pi \nu = \dfrac{2\pi}{T} \]
where \(\nu\) is frequency and period (T) is the amount of time for the wave to travel one wavelength.
Interference
An important property of waves is the ability to combine with other waves. There are two type of interference: constructive and destructive. Constructive interference occurs when two or more waves are in phase and and their displacements add to produce a higher amplitude. On the contrary, destructive interference occurs when two or more waves are out of phase and their displacements negate each other to produce lower amplitude.
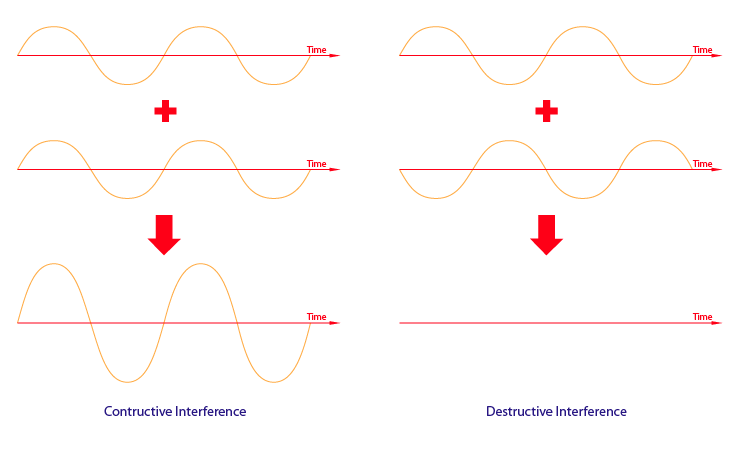
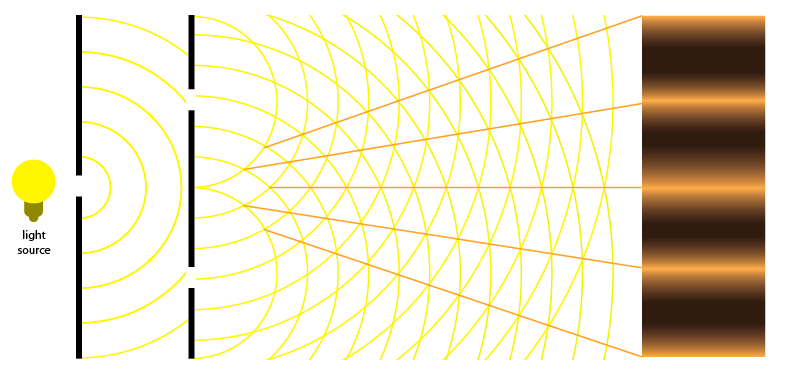
Figure 9 & 10: Constructive and Destructive Interference
Interference can be demonstrated effectively through the double slit experiment. This experiment consists of a light source pointing toward a plate with one slit and a second plate with two slits. As the light travels through the slits, we notice bands of alternating intensity on the wall behind the second plate. The banding in the middle is the most intense because the two waves are perfectly in phase at that point and thus constructively interfere. The dark bands are caused by out of phase waves which result in destructive interference. This is why you observe nodes on figure 4. In a similar way, if electrons are used instead of light, electrons will be represented both as waves and particles.
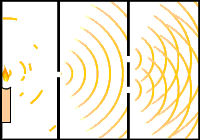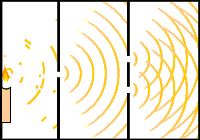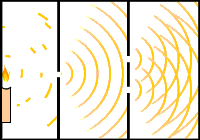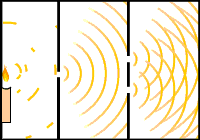
Fig. 11 & 12: Double-Slit Interference Experiment
Wave-Particle Duality
Electromagnetic radiation can either acts as a wave or a particle, a photon. As a wave, it is represented by velocity, wavelength, and frequency. Light is an EM wave since the speed of EM waves is the same as the speed of light. As a particle, EM is represented as a photon, which transports energy. When a photon is absorbed, the electron can be moved up or down an energy level. When it moves up, it absorbs energy, when it moves down, energy is released. Thus, since each atom has its own distinct set of energy levels, each element emits and absorbs different frequencies. Photons with higher energies produce shorter wavelengths and photons with lower energies produce longer wavelengths.
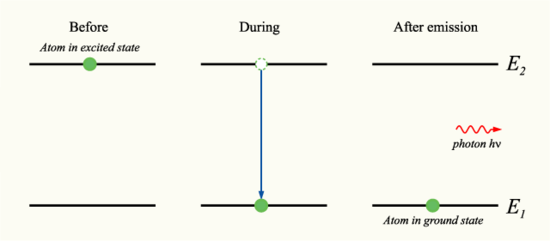
Fig. 13: Photon Before and After Emission
Ionizing and Non-Ionizing Radiation
Electromagnetic Radiation is also categorized into two groups based, ionizing and non-ionizing, on the severity of the radiation. Ionizing radiation holds a great amount of energy to remove electrons and cause the matter to become ionized. Thus, higher frequency waves such as the X-rays and gamma-rays have ionizing radiation. However, lower frequency waves such as radio waves, do not have ionizing radiation and are grouped as non-ionizing.
Electromagnetic Radiation and Temperature
Electromagnetic radiation released is related to the temperature of the body. Stephan-Boltzmann Law says that if this body is a black body, one which perfectly absorbs and emits radiation, the radiation released is equal to the temperature raised to the fourth power. Therefore, as temperature increases, the amount of radiation released increases greatly. Objects that release radiation very well also absorb radiation at certain wavelengths very well. This is explained by the Kirchhoff’s Law. Wavelengths are also related to temperature. As the temperature increases, the wavelength of maximum emission decreases.
Problems
- What is the wavelength of a wave with a frequency of 4.28 Hz?
- What is the frequency of a wave with a wavelength of 200 cm?
- What is the frequency of a wave with a wavelength of 500 pm?
- What is the wavelength of a wave with a frequency of 2.998 × 105 Hz?
- A radio transmits a frequency of 100 Hz. What is the wavelength of this wave?
Answers:
- 700m
- 1.5 × 108 Hz
- 4.0 × 1017 Hz
- 100m
- 2.998 × 106 m
References
- Atkins, Peter and Julio de Paula. Physical Chemistry for the Life Sciences. New York: Oxford University Press, 2006.
- Chang, Raymond. Physical Chemistry for the Biosciences. USA: University Science Books, 2005.
- McQuarrie, Donald and Simon, John. Physical Chemistry: A Molecular Approach. Sausalito, CA: University Science Books, 1997.
- Price, Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Problems in Physical Chemistry for Biochemists. R. G. Ratcliffe. New York: Oxford University Press, 1997.