10.28: Hydrogen Atom Calculation Assuming the Electron is a Particle in a Sphere of Radius R
- Page ID
- 136978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Trial wave function:
\[ \Phi (r, R) := \frac{1}{ \sqrt{2 \pi R}} \frac{ \sin ( \frac{ \pi r}{R})}{r} \nonumber \]
Integral:
\[ \int_{0}^{ \infty} \blacksquare 4 \pi r^2 dr \nonumber \]
Kinetic energy operator:
\[ T = \frac{1}{2r} \frac{d^2}{dr^2} (r \blacksquare ) \nonumber \]
Potential energy operator:
\[ V = \frac{1}{r} \nonumber \]
Demonstrate the wave function is normalized.
Set up the variational energy integral.
\[ E(R) := \int_{0}^{R} \Phi (r, R) [ \frac{-1}{2r} \frac{d^2}{dr^2} (r \Phi (r, R))] 4 \pi r^2 dr + \int_{0}^{R} \Phi (r, R) \frac{-1}{r} \Phi (r, R) 4 \pi r^2 dr] \nonumber \]
Minimize the energy with respect to the variational parameter R.
R := 1 R := Minimize(E, R) R = 4.049 E(R) = -0.301
The exact ground state energy for the hydrogen atom is -.5 Eh. Calculate the percent error.
\[ \frac{-.5 - E(R)}{-.5} = 39.793 \nonumber \]
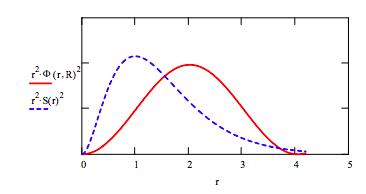
Compare optimized trial wave function with the exact solution by plotting the radial distribution functions.
\[ S(r) := \frac{1}{ \sqrt{ \pi}} exp(-r)~~~r := 0,.02..4.2 \nonumber \]