10.24: Variational Calculation on Helium Using a Hydrogenic Wavefunction
- Page ID
- 136973
Gaussian trial wavefunction:
\[ \psi (r, \beta ) := \sqrt{ \frac{ \beta^3}{ \pi}} exp(- \beta r) \nonumber \]
Demonstrate the wavefunction is normalized.
\[ \int_{0}^{ \infty} \psi (r, \beta )^2 4 \pi r^2 dr |_{simplify}^{assume,~ \beta > 0} \rightarrow 1 \nonumber \]
The terms contributing to the total electronic energy of the helium atom are the kinetic energy of each electron, each electron's interaction with the nucleus, and the interaction of electrons with each other.
Calculate kinetic energy:
\[ 2 \int_{0}^{ \infty} \psi (x, \beta )^2 \left[ \frac{-1}{2} \frac{d^2}{dx^2} (r \psi ( r, \beta )) \right]4 \pi r^2 dr |_{simplify}^{assume,~ \beta >0} \rightarrow \beta ^2 \nonumber \]
Calculate electron-nucleus potential energy:
- a. Calculate the electric potential of one of the electrons in the presence of the other: \[ \frac{1}{r} \int_{0}^{r} \psi (x, \beta )^2 4 \pi x^2 dx + \int_{r}^{ \infty} \frac{ \psi (x, \beta )^2 4 \pi x^2}{x} dx |_{simplify}^{assume,~ \beta >0} \rightarrow \frac{- [r \beta e^{(-2) r \beta} + e^{(-2) r \beta} - 1]}{r} \nonumber \]
- b. Calculate the electron-electron potential energy using result of part a: \[ \int_{ \infty}^{r} \psi (x, \beta )^2 \frac{- [r \beta e^{(-2) r \beta} + e^{(-2) r \beta} - 1]}{r} 4 \pi r^2 dr |_{simplify}^{assume,~ \beta > 0 \rightarrow \frac{5}{8} \beta} \nonumber \]
Write the equation for the total electronic energy in terms of the variational parameter \(β\) and minimize the energy with respect to \(β\):
\[ E( \beta ) := \beta ^2 - 4 \beta + \frac{5}{8} \beta~~~ \beta := \frac{d}{d \beta} E( \beta ) = 0~solve,~ \beta \rightarrow \frac{27}{16}~~~ E( \beta ) = -2.848 \nonumber \]
Compare the variational calculation to the Hartree‐Fock limit: (\(E_{HF} \approx −2.8617\))
\[ \dfrac{E_{HF} - E( \beta )}{E_{HF}} = 0.491 \% \nonumber \]
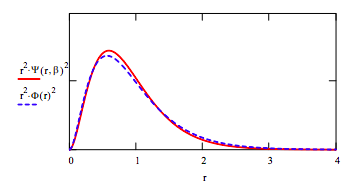
Compare optimized trial wavefunction with the Hartree‐Fock wavefunction by plotting the radial distribution functions.
\[ \Phi (r) = 0.75738 \exp(-1.430r) + 0.43658 \exp(-2.4415r) + 0.17295 \exp(-4.0996r)- 0.02730\exp(-6.4843r) + 0.06675\exp(-7.978r) \nonumber \]