Solubility
- Page ID
- 2356
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the general properties of ionic compounds is water solubility. The oceans are solutions of salt in water. In a mixture, two or more materials are mixed together but they remain essentially separate, like sand and water. The sand can be easily distinguished from the water, because even if a mixture of the two is shaken it will spontaneously separate over time.
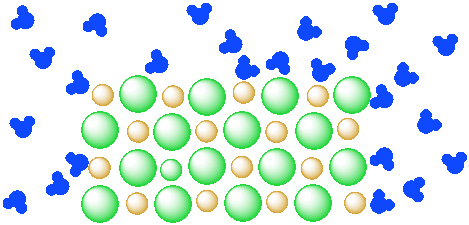
In a suspension, one or more materials is mixed into a liquid, and the mixture becomes somewhat homogeneous. Instead of having easily identifiable layers, the liquid has a uniform appearance throughout. However, suspensions are generally cloudy liquids. Milk is a suspension containing water, fats and proteins. They may settle out into separate layers eventually, but it takes time.
In a solution, one or more materials is mixed into a liquid, and the mixture becomes a completely homogeneous liquid. Solutions are transparent, not cloudy, and can be colored or colorless. Saltwater, an example of a solution, is diagrammed in the figure below.
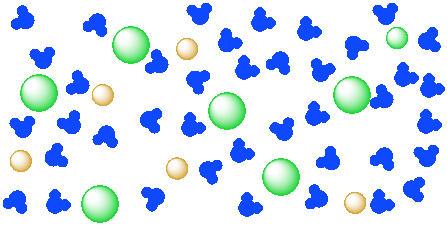
Pieces of salt are not visible in the solution; the salt particles are too small. The salt is separated into individual ions, surrounded by water molecules. This change from Figure IC4.3 to Figure IC4.2 is not instantaneous upon adding salt to water; stirring is required to produce the solution.

Eventually more of the salt dissolves in the water, as shown:
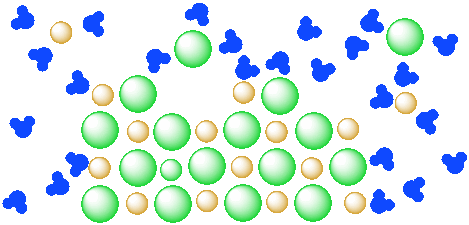
If enough salt is added, the system might come to "equilibrium": the water has dissolved all of the salt that it can, so the rest of the salt remains solid. This equilibrium is "dynamic": ions are dissolved in the water at the same time that ions are deposited from solution into the solid state. However, the overall ratio of dissolved ions to water stays the same.
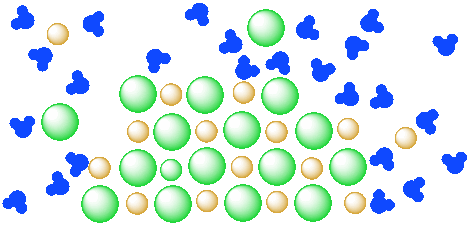
Problem IC4.1.
Consider further the idea that a given amount of water is only able to dissolve a specific amount of salt.
- In the diagram above, how many water molecules are there?
- How many units of salt (an anion and a cation) are dissolved?
- If there were only a dozen water molecules present, how many units of salt would dissolve?
- If a hundred water molecules were present, how many units of salt would dissolve?
Why do salts dissolve in water? Water is a molecular compound; the atoms are directly attached to each other, rather than being ions that are attracted to each other. Because of electronegativity differences, the oxygen atom in water has a partial negative charge and the hydrogen atoms have partial positive charges. Ionic compounds can dissolve in polar liquids like water because the ions are attracted to either the positive or negative part of the molecule.
There is a sort of tug-of-war involved with species dissolved in water. The water pulls individual ions away from the solid. The solid is pulling individual ions back out of the water. There exists an equilibrium based on how strongly the water attracts the ions, versus how strong the ionic solid attracts the ions.
It is possible to predict varying degrees of solubility in water for different ionic compounds using the principles of Coulomb's law. The smaller the ions, the closer together they are, and the harder it is for the water molecules to pull the ions away from each other.
Problem IC4.2.
Predict which of the following pairs should be more soluble in water, based on the Coulombic attraction between ions.
- LiF or NaF
- NaK or KF
- BeO or LiF
Problem IC4.3.
Although lithium fluoride and magnesium oxide contain cations and anions of roughly the same size, lithium fluoride is much more soluble in water (2.7 g/L) than magnesium oxide (0.087 g/L) at room temperature. Propose a reason why.
The trends in melting points in ionic compounds are more complicated with regard to solubility. The water solubility of alkali chlorides does not follow a simple trend (as shown in Table IC4.1).
Table IC4.1 Water solubility among alkali chlorides.
| Compound | Water Solubility in g/L at 20oC |
| LiCl | 83 |
| NaCl | 359 |
| KCl | 344 |
Lithium chloride is the least water-soluble of the three compounds. This is feasible, as the lithium ions are small and the attraction for the chloride would be stronger over that shorter distance. However, potassium chloride would be expected to be the most soluble of the three compounds, and it is slightly less soluble than sodium chloride.
Problem IC4.4.
Propose an explanation for why the water solubility of the alkali chlorides does not simply increase as the cation gets larger.
If the halide is varied is used, similar trends are observed:.
Table IC4.2 Water solubility among lithium halides.
| Compound | Water Solubility in g/L at 20oC |
|---|---|
| LiCl | 83 |
| LiBr | 166 |
| LiI | 150 |
Again, it is unsurprising that the lithium chloride is the least soluble, but the most soluble is the lithium bromide, not the lithium iodide.
This type of behavior indicates that there is more than one factor influencing the phenomenon of interest. In the case of solubility, there are several other factors, some of which are more complicated. One simply involves the fact that there are two interactions to consider. The dissolution of these compounds requires more than simply overcoming the attraction of the ionic solid for individual ions, as does melting: the attraction of the water to the ion must also be considered. That attraction is also governed by Coulomb's Law. At some stage, there is a tipping point, when the factors that increase attraction between the ions also increase the attraction between the ion and the water. One or the other of these factors becomes the dominant influence under different circumstances.
- Several interactions are involved in dissolution.
- Cation-anion attraction is just one of these interactions.
- Cation-water and anion-water interactions are important, too.
- Water-water interactions also play a role.


