Chemical Bond
- Page ID
- 32709
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain material structures in terms of chemical bonds.
- Describe various concepts developed for chemical bonds.
- Expand the concept of chemical bond (to be innovative, and creative).
- Describe the octet rule and apply it to write Lewis dot structures for ions and molecules.
Chemical Bond
Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds. Although electrons of one atom repel electrons of another, the repulsion is relatively small. So is the repulsion between atomic nuclei.
Various theories regarding chemical bonds have been proposed over the past 300 years, during which our interpretation of the world has also changed. Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well.
While reading this page, you learn new concepts such as bond length, bond energy, bond order, covalent bond, ionic bond, polar and non-polar bond etc. These concepts help you understand the material world at the molecular level.
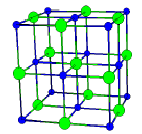 Chemical bonds between identical atoms such as those in \(\ce{H2}\), \(\ce{N2}\), and \(\ce{O2}\) are called covalent bonds, in which the bonding electrons are shared. In ionic compounds, such as \(\ce{NaCl}\), the ions gather and arrange in a systematic fashion to form a solid. The arrangement of (blue) \(\ce{Na+}\) and (green) \(\ce{Cl-}\) ions in a solid is shown in on the right here. The attraction force between ions is called ionic bonding. Metals such as sodium, copper, gold, iron etc. have special properties such as being good electric conductors. Electrons in these solids move freely throughout the entire solid, and the forces holding atoms together are called metallic bonds. To some extent, metals are ions submerged in electrons.
Chemical bonds between identical atoms such as those in \(\ce{H2}\), \(\ce{N2}\), and \(\ce{O2}\) are called covalent bonds, in which the bonding electrons are shared. In ionic compounds, such as \(\ce{NaCl}\), the ions gather and arrange in a systematic fashion to form a solid. The arrangement of (blue) \(\ce{Na+}\) and (green) \(\ce{Cl-}\) ions in a solid is shown in on the right here. The attraction force between ions is called ionic bonding. Metals such as sodium, copper, gold, iron etc. have special properties such as being good electric conductors. Electrons in these solids move freely throughout the entire solid, and the forces holding atoms together are called metallic bonds. To some extent, metals are ions submerged in electrons.
A Brief Past on Chemical Bond Concepts
Various concepts or theories have been proposed to explain the formation of compounds. In particular, chemical bonds were proposed to explain why and how one element reacted with another element. In 1852, E. Frankland proposed the concept of valence. He suggested that each element formed compounds with definite amounts of other elements due to a valence connection. Each element has a definite number of valence.
Five years later, F.A. Kekule and others proposed a valence of 4 for carbon. Lines were used to represent valence, and this helped the development of organic chemistry. The structure of benzene was often quoted as an achievement in this development. More than 10 years later, J.H. van 't Hoff and le Bel proposed the tetrahedral arrangement for the four valences around the carbon. These theory helped chemists to describe many organic compounds. In the meantime, chemical bonds were thought to be electric in nature. Since electrons have not been discovered as the negative charge carriers, they were thought to be involved in chemical bonds.
Following the discoveries of electrons by J.J. Thomson and R. A. Millikan, G.N. Lewis proposed to use dots to represent valence electrons. His dots made the valence electrons visible to chemists, and he has been credited as the originator of modern bonding theory. He published a book, in 1923, called Valence and the Structure of Atoms and Molecules.
X-ray diffractions by crystal allow us to calculate details of bond length and bond angles. Using computers, we are able to generate images of molecules from the data provided by X-ray diffraction studies. These data prompted Linus Pauling to look at The Nature of the Chemical Bond, a book that introduced many new concepts such as the resonance, electronegativity, ionic bond, and covalent bond. In England, N.V. Sidgwick and H.E. Powell paid their attention to the lone pairs in a molecule. They developed the valence bond theory, the VSEPR (Valence Shell Electron Pair Repulsion) theory. The application of quantum theory to chemical bonding gave birth to a molecular orbital theory. In this and the few following modules, we will look at some of these concepts in detail.
Lewis Dot Structures
For the elements in the 2nd and 3rd periods, the number of valence electrons range from 1 to 8. Lewis dot structure for them are as indicated:
\(\mathrm{
\overset{\Large{.}}Li \hspace{20px}
\underset{\Large{.}}{\overset{\Large{.}}Be}\hspace{20px}
\cdot \overset{\Large{.}}{B} \cdot\hspace{20px}
\cdot \underset{\Large{.}}{\overset{\Large{.}}{C}} \cdot\hspace{20px}
\cdot \underset{\Large{.}}{\overset{\Large{.}}{N}} :\hspace{20px}
: \underset{\Large{.}}{\overset{\Large{.}}{O}} :\hspace{20px}
: \underset{\Large{.}}{\overset{\Large{..}}{F}} :\hspace{20px}
: \underset{\Large{..}}{\overset{\Large{..}}{Ne}} :}
\)
Using dots, Lewis made the valence electron visible. The stability of noble gases is now associated with the 8 valence electrons around them. The stability of 8 valence electrons led him to conclude that all elements strive to acquire 8 electrons in the valence shell, and the chemical reaction takes place due to elements trying to get 8 electrons. This is the octet rule. For the hydrogen and helium atoms, 2 electrons instead of 8 are required.
For example, the octet rule applies to the following molecules:
| \(\mathrm{H : H}\) (2 electrons) |
\(\mathrm{H : \underset{\Large{..}}{\overset{\Large{..}}{O}} : H}\) |
\(\mathrm{H : \underset{\Large{..}}{\overset{\Large{..}}{F}} :}\) |
\(\mathrm{H : \underset{\Large{..}}{\overset{\Large{\underset{\huge{..}}H}}{N}} : H}\) | \(\mathrm{H : \underset{\Large{\overset{\huge{..}}H}}{\overset{\Large{\underset{\huge{..}}H}}{C}} : H}\) | \(\mathrm{: N ::: N :}\) | \(\mathrm{: \overset{\Large{..}}O :: \overset{\Large{..}}O :}\) | \(\mathrm{: \overset{\Large{..}}F : \overset{\Large{..}}F :}\) | \(\mathrm{: \overset{\Large{..}}O :: C :: \overset{\Large{..}}O : }\) |
|---|
To draw a Lewis dot structure, all the valence electrons are represented. A good way is to draw a type of dot for the valence electrons of one atom different from types in another. To do this on the computer screen using only type fonts is difficult, but you should draw a few by hand on paper.
When a dash is used to represent a bond, it represents a pair of electrons. Thus, in the following representations, a dash represents two electrons, bonding or lone pairs.
_ _ _
:S=O: :O:H :O:H
| _ | | -
O :O:S:O: :O:S:O:
" | " " | "
:O:H :O:H
" "
|
These structures satisfy the octet rule. Note the two ways of drawing the structures of \(\ce{H2SO4}\). |
Exceptions to the Octet Rule
Elements in the 3rd and higher periods may have more than 8 valence electrons. A possible explanation for this is to say that these atoms have d-type atomic orbitals to accommodate more than 8 electrons. In the following molecules, the number of valence electrons in the central atoms are as indicated:
| Molecule | \(\ce{SF6}\) | \(\ce{PCl5}\) | \(\ce{ICl3}\) | \(\ce{XeF4}\) |
|---|---|---|---|---|
| No. of valence electrons for central atom |
12 | 10 | 10 | 12 |
OH | O=S=O | OH |
Draw the Lewis dot structures for the above molecules, and count the number of valence electrons for the central atoms. For \(\ce{H2SO4}\), the \(\ce{S}\) atom has 12 electrons in the structure shown on your right. Each dash represents a chemical bond, which has two electrons. There is a total of 6 bonds around the \(\ce{S}\) atom, and therefore 12 electrons.
When \(\ce{B}\), \(\ce{Be}\), and some metals are the central atoms, they have less than 8 valence electrons. The following compounds do form, but the octet rule is not satisfied. These are electron deficient molecules.
| Molecule | \(\ce{BeCl2}\) | \(\ce{BF3}\) | \(\ce{BCl3}\) | \(\ce{SnCl2}\) |
|---|---|---|---|---|
| No. of valence electrons for central atom |
4 | 6 | 6 | 6 |
Another case of exception to the octet rule is molecules with an odd number of valence electrons. For example:
| \(\ce{NO}\) | \(\ce{NO2}\) | \(\ce{ClO2}\) | |
|---|---|---|---|
| No. of valence electrons for central atom |
11 | 17 | 19 |
Isoelectronic Molecules and Ions
Counting the number of valence electrons often helps us understand the formation of many molecules and ions. For example, all the following molecules have 11 valence electrons:
| \(\ce{NO}\) | \(\ce{CO-}\) | \(\ce{O2+}\) | \(\ce{N2-}\) |
|---|
The charged molecules do exist under special circumstances.
The molecules of \(\ce{O2}\) are paramagnetic, and thus, they have unpaired electrons. The first dot structure does not agree with this observed fact, but the second one does. However, the second one does not obey the octet rule.
| \(\mathrm{\overset{\Large{.\,.}}{: O :}\overset{\Large{.\,.}}{: O :}}\) | \(\mathrm{:\overset{\Large .}{O}:\,:\,:\overset{\Large .}{O}:}\) |
|---|---|
| No unpaired electron |
Violates octet rule |
Later, you will learn that the molecular orbital (MO) theory provides a good explanation for the electronic configuration for \(\ce{O2}\).
Confidence Building Questions
- In 1852, E. Frankland proposed the concept of valence. What is the meaning of valence in this early stage?
- Number of valence electrons.
- Number of atoms.
- Number of chemical bonds an element can form.
- Number of sticks an atom have.
Hint: c. Number of chemical bonds an element can form.
Skill: Describe a concept. Kekule applied this concept later and suggested four valence for carbon.
- What do the dots in Lewis dot structures represent?
Skill: Describe the Lewis dot structure
The dots represent valence electrons, including those not involved in bonding. Lewis dots made the unshared electrons visible. - How many valence electrons do the atoms of oxygen have?
Skill: Describe the properties of an element based on its group.
Oxygen is the first element of group 6 in the periodic table. How about sulfur, \(\ce{S}\)? - Which one of the following molecules does not satisfy the octet rule?
\(\ce{CO2}\), \(\ce{CO3^2-}\), \(\ce{N2O}\), \(\ce{NO2}\), \(\ce{NO}\)Discussion: Which one of these has an odd number of electrons? The dot structure for \(\ce{N2O}\) is \(\mathrm{:: N : N : \overset{\Large{.\,.}}{O} :::}\)
- Which of the following molecules satisfy the octet rule?
\(\ce{BF3}\), \(\ce{BeCl2}\), \(\ce{SnCl2}\), \(\ce{CO}\), \(\ce{SF6}\), \(\ce{XeF4}\), \(\ce{NO}\)Skill: For \(\ce{CO}\), you have \(\mathrm{:C:\,:\,:O:}\) as the dot structure. The molecule \(\ce{NO}\) has one more electron than that of \(\ce{CO}\).
- Which of the following molecules has an odd number of total valence electrons?
\(\ce{CO}\), \(\ce{CO2}\), \(\ce{N2O}\), \(\ce{NH3}\), \(\ce{H2O}\), \(\ce{NO2}\)Skill: Elements in the second period are: \(\ce{Li}\), \(\ce{Be}\), \(\ce{B}\), \(\ce{C}\), \(\ce{N}\), \(\ce{O}\), \(\ce{F}\), \(\ce{Ne}\).
Compare the number of total valence electrons between \(\ce{N2O}\) and \(\ce{NO2}\).
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

