2.17: Atomic Stability - Mathcad Version
- Page ID
- 155625
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Neils Bohr once observed that from the perspective of classical physics the stability of matter was a pure miracle. The problem, of course, is that two of the basic building blocks of matter are oppositely charged - the electron and the proton. Given Coulomb's Law the troubling question is what keeps them from coalescing?
Quantum mechanics is considered by many to be an abstract and esoteric science that doesn't have much to do with everyday life. Yet it provides an explanation for atomic and molecular stability, and classical physics fails at that task. Thus, to achieve some understanding of one of the basic facts about the macro-world requires quantum mechanical concepts and tools.
The issue of atomic stability will be explored with a quantum mechanical analysis of the two simplest elements in the periodic table - hydrogen and helium. They are also the two most abundant elements in the universe. Schrödinger's equation can be solved exactly for the hydrogen atom, but approximate methods are required for the helium atom. In this study, the variational method will be used for both hydrogen and helium.
Variational Method for the Hydrogen Atom
Normalized trial wave function:
\[ \Psi ( \alpha,~r) = \sqrt{ \frac{ \alpha^3}{ \pi}} \text{exp} ( - \alpha,~r)
α is a scale-factor that controls the size of the wave function.
Integral:
\[ \int_0^{ \infty} \blacksquare 4 \pi r^2 dr \nonumber \]
Kinetic energy operator:
\[ - \frac{1}{2r} \frac{d^2}{dr^2} ( r \blacksquare ) \nonumber \]
Potential energy operator:
\[ \frac{-Z}{r} \blacksquare \nonumber \]
Demonstrate that the trial function is normalized:
\[ \int_0^{ \infty} \Psi ( \alpha,~R)^2 4 \pi r^2 \text{dr assume, } \alpha > 0 \rightarrow 1 \nonumber \]
Plot trial wave function for several values of α, the variational parameter:
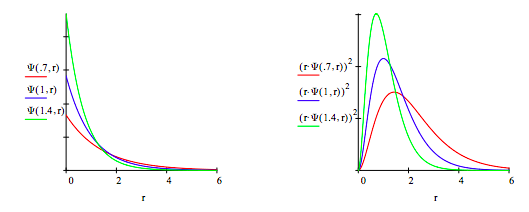
Calculate the average value of the kinetic energy of the electron:
\[ T ( \alpha ) = \int_0^{ \infty} \Psi ( \alpha,~ r) - \frac{1}{2r} \frac{d^2}{dr^2} (r \Psi ( \alpha,~r)) 4 \pi r^2 \text{ dr assume, } \alpha > 0 \rightarrow \frac{ \alpha^2}{2} \nonumber \]
Calculate the average value of the potential energy of the electron:
\[ V( \alpha,~ Z) = \int_0 ^{ \infty} \Psi ( \alpha,~r) - \frac{Z}{r} \Psi ( \alpha,~r) 4 \pi r^2 \text{ dr assume, } \alpha > 0 \rightarrow - Z \alpha \nonumber \]
Calculate R, the average distance of the electron from the nucleus:
\[ R ( \alpha ) = \int_0 ^{ \infty} \Psi ( \alpha,~r) r \Psi ( \alpha,~r) 4 \pi r^2 \text{ dr assume, } \alpha > 0 \rightarrow \frac{3}{2 \alpha} \nonumber \]
From this we find that:
\[ E ( \alpha ) = T ( \alpha ) + V( \alpha,~Z) = \frac{ \alpha^2}{2} - \alpha Z \nonumber \]
But from above we knew:
\[ \alpha = \frac{3}{2R} \nonumber \]
This allows us to express the total energy and its components in terms of R the average distance of the electron from the nucleus.
Total energy:
\[ \begin{array}{c|c} \text{E(R, Z)} = \frac{ \alpha^2}{2} - \alpha Z &_{ \text{expand}}^{ \text{substitute, } \alpha = \frac{3}{2R}} \rightarrow \frac{9}{8R^2} - \frac{3Z}{2R} \end{array} \nonumber \]
Electron kinetic energy:
\[ T_E (R) = \frac{9}{8R^2} \nonumber \]
Electron-nucleus potential energy:
\[ V_{NE} (R,~Z) = \frac{-3Z}{2R} \nonumber \]
E, TE and VNE are graphed versus R for the hydrogen atom (Z = 1): R = 0, .01 ... 8
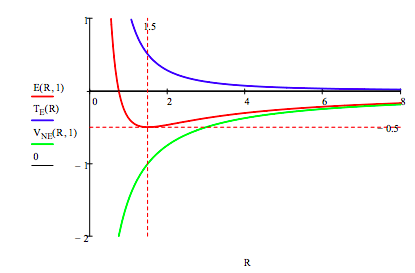
The hydrogen atom ground-state energy is determined by minimizing its energy with respect to R:
\[ \begin{matrix} \text{R=R} & \text{R} = \frac{d}{dR} \text{E(R 1) = solve, R} \rightarrow \frac{3}{2} & \text{E(R, 1) = -0.5} \end{matrix} \nonumber \]
Imagine a hydrogen atom forming as an electron approaches a proton from a great distance. The electron is drawn toward the proton by the Coulombic attractive interaction between the two opposite charges and the potential energy decreases like -1/R. The attractive potential energy interaction confines the electron to a smaller volume and the kinetic energy increases as 1/R2. Thus the kinetic energy goes to positive infinity faster than the potential energy goes to negative infinity and a total energy minimum (ground state) is achieved at R = 1.5, as shown in the figure above.
The electron does not collapse into (coalesce with) the proton under the influence of the attractive Coulombic interaction because of the repulsive effect of the confinement energy - that is, kinetic energy. Kinetic energy, therefore, is at the heart of understanding atomic stability.
Variational Method for the Helium Atom
Now we will proceed to the He atom. There are five contributions to the total electronic energy: kinetic energy of each electron, the interaction of each electron with the nucleus, and the electron-electron interaction.The only new term is the last, electron-electron potential energy. It is evaluated as follows for two electrons in 1s orbitals.
The electrostatic potential at r due to electron 1 is:
\[ \begin{array}{c|c} \Phi ( \alpha,~r) = \frac{1}{r} \int_0^r \Psi ( \alpha,~ x)^2 4 \pi x^2 dx + \int_r^{ \infty} \frac{ \Psi ( \alpha,~x)^2 4 \pi x^2}{x} dx & _{ \text{simplify}} ^{ \text{assume, } \alpha > 0} \rightarrow - \frac{e^{-2 \alpha r} + \alpha r e^{-2 \alpha r} - 1}{r} \end{array} \nonumber \]
The electrostatic interaction between the two electrons is:
\[ \begin{array}{c|c} V_{EE} = \int_0 ^{ \infty} \Phi ( \alpha,~r) \Psi ( \alpha,~r)^2 4 \pi r^2 dr & _{ \text{simplify}}^{ \text{assume, } \textcolor{red}{ \alpha} >0} \rightarrow \frac{5 \alpha}{8} \end{array} \nonumber \]
In terms of R, the electron-electron potential energy is:
\[ V_{EE} (R) = \frac{15}{16R} \nonumber \]
\[ \begin{matrix} Z = 2 & E_{He} (R) = 2T_E (R) + 2 V_{NE} (R,~Z) + V_{EE} (R) & V(R) = 2 V_{NE} (R,~Z) + V_{EE} (R) \end{matrix} \nonumber \]
The various contributions to the total electronic energy of the helium atom are plotted below. R = 0, .01 .. 4
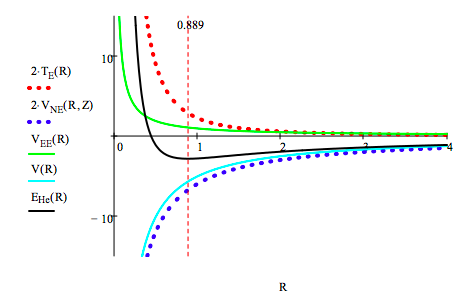
The helium atom ground-state energy is determined by minimizing its energy with respect to R:
\[ \begin{matrix} \text{R = R} & \text{R} = \frac{d}{dR} E_{He} (R) = \text{0 solve, R} \rightarrow \frac{8}{9} & \text{R} = 0.889 & E_{He} (R) = -2.848 \end{matrix} \nonumber \]
Graphing EHe vs. reveals again that kinetic energy (confinement energy) is the key to atomic stability. Several things should be noted in the graph shown above. First, that when the total energy minimum is achieved VNE and V (VNE + VEE) are still in a steep decline. This is a strong indication that VEE is really a rather feeble contribution to the total energy, increasing significantly only long after the energy minimum has been attained. Thus electron-electron repulsion cannot be used to explain atomic stability. The graph above clearly shows that on the basis of classical electrostatic interactions, the electron should collapse into the nucleus. This is prevented by the kinetic energy term for the same reasons as were given for the hydrogen atom.
Unfortunately chemists give too much significance to electron-electron repulsion (VSEPR for example) when it is really the least important term in the Hamiltonian energy operator. And to make matters worse they completely ignore kinetic energy as an important factor in atomic and molecular phenomena. It is becoming increasing clear in the current literature that many traditional explanations for chemical phenomena based exclusively on electrostatic arguments are in need of critical re-examination.

