9.8: Molecular-Orbital Theory Does not Predict a Stable Diatomic Helium Molecule
- Page ID
- 13460
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Using bond order as a metric for the existance of molecules
Bond Order
In the Lewis electron structures, the number of electron pairs holding two atoms together was called the bond order. Within the molecular orbital approach, bond order is defined as one-half the net number of bonding electrons:
\[ \text{bond order}=\dfrac{\text{number of bonding electrons} - \text{number of antibonding electrons}}{2} \label{9.8.1} \]
To calculate the bond order of \(H_2\), we know that the \(σ_{1s}\) (bonding) molecular orbital contains two electrons, while the \( \sigma _{1s}^{\star } \) (antibonding) molecular orbital is empty. The bond order of \(H_2\) is therefore
\[ \text{bond order}=\dfrac{2-0}{2}=1 \label{9.8.2} \]
This result corresponds to the single covalent bond; double and triple bonds contain four or six electrons, respectively, and correspond to bond orders of 2 and 3.
We can use energy-level diagrams to describe the bonding in other pairs of atoms and ions where n = 1, such as the H2+ ion, the He2+ ion, and the He2 molecule. Again, we fill the lowest-energy molecular orbitals first while being sure not to violate the Pauli principle or Hund's Rules.
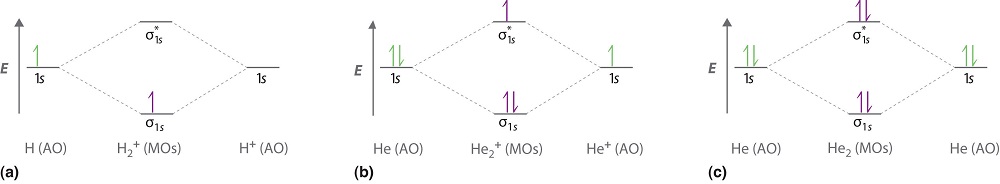
Figure \(\PageIndex{1a}\) shows the energy-level diagram for the H2+ ion, which contains two protons and only one electron. The single electron occupies the \(σ_{1s}\) bonding molecular orbital, giving a (σ1s)1 electron configuration. The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (via Equation \ref{9.8.1})
\[\text{bond order}=\dfrac{1-0}{2}=1/2 \nonumber \]
Because the bond order is greater than zero, the H2+ ion should be more stable than an isolated H atom and a proton. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+. With a bond order of only 1/2 the bond in H2+ should be weaker than in the H2 molecule, and the H–H bond should be longer. As shown in Table 9.8.1 , these predictions agree with the experimental data.
| Species | Electron Configuration | Bond Order | Bond Length (pm) | Bond Energy (kJ/mol) |
|---|---|---|---|---|
| H2+ | \((σ_{1s})^1\) | 1/2 | 106 | 269 |
| H2 | \((σ_{1s})^2\) | 1 | 74 | 436 |
| He2+ | \( \left (\sigma _{1s} \right )^{2}\left (\sigma _{1s}^{\star } \right )^{1} \) | 1/2 | 108 | 251 |
| He2 | \( \left (\sigma _{1s} \right )^{2}\left (\sigma _{1s}^{\star } \right )^{2} \) | 0 | 5,500 | \(4.6 \times 10^{−5}\) |
Figure \(\PageIndex{1b}\) is the molecular orbital energy-level diagram for \(\ce{He_2^{2+}}\). This ion has a total of three valence electrons. Because the first two electrons completely fill the \(σ_{1s}\) molecular orbital, the Pauli principle states that the third electron must be in the \( \sigma _{1s}^{\star} \) antibonding orbital, giving a \( \left (\sigma _{1s} \right )^{2}\left (\sigma _{1s}^{\star } \right )^{1} \) electron configuration. This electron configuration gives a bond order (via Equation \ref{9.8.1}) of
\[\text{bond order}=\dfrac{2-1}{2}=1/2 \nonumber \]
As with H2+, the He2+ ion should be stable, but the He–He bond should be weaker and longer than in H2. In fact, the He2+ ion can be prepared, and its properties are consistent with our predictions (Table 9.8.1 ).
Use a molecular orbital energy-level diagrams to predict the bond order and stability of the \(\ce{He_2^{2+}}\) ion.
Given: chemical species
Asked for: molecular orbital energy-level diagram, bond order, and stability
Strategy:
- Combine the two He valence atomic orbitals to produce bonding and antibonding molecular orbitals. Draw the molecular orbital energy-level diagram for the system.
- Determine the total number of valence electrons in the He22+ ion. Fill the molecular orbitals in the energy-level diagram beginning with the orbital with the lowest energy. Be sure to obey the Pauli principle and Hund’s rule while doing so.
- Calculate the bond order and predict whether the species is stable.
Solution:
A Two He 1s atomic orbitals combine to give two molecular orbitals: a \(σ_{1s}\) bonding orbital at lower energy than the atomic orbitals and a \( \sigma _{1s}^{\star } \) antibonding orbital at higher energy. The bonding in any diatomic molecule with two He atoms can be described using the following molecular orbital diagram:
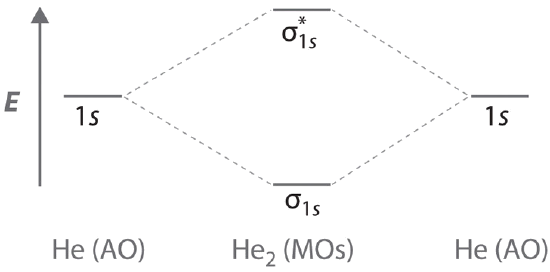
B The He22+ ion has only two valence electrons (two from each He atom minus two for the +2 charge). We can also view He22+ as being formed from two He+ ions, each of which has a single valence electron in the 1s atomic orbital. We can now fill the molecular orbital diagram:
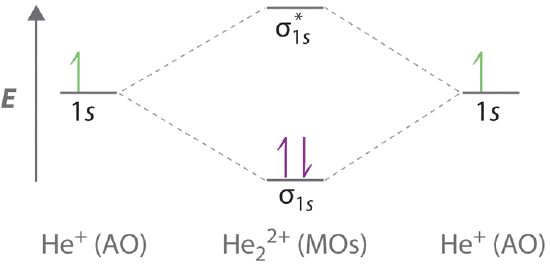
The two electrons occupy the lowest-energy molecular orbital, which is the bonding (\(σ_{1s}\)) orbital, giving a (σ1s)2 electron configuration. To avoid violating the Pauli principle, the electron spins must be paired.
C So the bond order is (via Equation \ref{9.8.1})
\[ \dfrac{2-0}{2} =1 \nonumber \]
He22+ is therefore predicted to contain a single He–He bond. Thus it should be a stable species.
Use a molecular orbital energy-level diagram to predict the valence-electron configuration and bond order of the \(\ce{H_2^{2−}}\) ion. Is this a stable species?
- Answer
-
\(\ce{H_2^{2−}}\) has a valence electron configuration of \( \left (\sigma _{1s} \right )^{2}\left (\sigma _{1s}^{\star } \right )^{2} \) with a bond order of 0. It is therefore predicted to be unstable.
The Helium Dimer
Finally, we examine the He2 molecule, formed from two He atoms with 1s2 electron configurations. Figure \(\PageIndex{1c}\) is the molecular orbital energy-level diagram for He2. With a total of four valence electrons, both the \( \sigma _{1s} \) bonding and \( \sigma _{1s}^{\star } \) antibonding orbitals must contain two electrons. This gives a \( \left (\sigma _{1s} \right )^{2}\left (\sigma _{1s}^{\star } \right )^{1} \) electron configuration, with a predicted bond order (via Equation \ref{9.8.1}) of
\[\text{bond order}= \dfrac{2 − 2}{2} = 0 \label{heliumdimer} \]
which indicates that the \(He_2\) molecule has no net covalent bond and is not a stable species.
The ability to track bond order to bond strength is due to the fact that the energy difference between the anti-bonding \( \sigma _{1s}^{\star } \) molecular orbital and the original \(1s\) atomic orbital is larger than the energy difference between the bonding \( \sigma _{1s} \) molecular orbital and the \(1s\) atomic orbitals. This was derived previously where the stabilization energy (\(\Delta E_{+}\)) of the \( \sigma _{1s} \) molecular orbital is less than the destabilization energy (\(\Delta E_{-}\)) of the anti-bonding \( \sigma _{1s}^{\star } \) molecular orbital:
\[ \begin{align} \Delta E_{\pm} &= E_{\pm} - E_H \\[4pt] &= \dfrac {e^2}{4\pi \epsilon _0 R} + \dfrac {J \pm K}{1 \pm S} \label {10.31} \end{align} \]
Hence, the anti-bonding \( \sigma _{1s}^{\star }\) molecular orbital is more destabilized relative to the atomic orbitals than the bonding \( \sigma _{1s} \) molecular orbital is stabilized relative to the \(1s\) atomic orbitals (\(E_H\).
The fact that the anti-bonding MO energy difference is larger than the bonding \( \sigma _{1s} \) molecular orbital energy difference is the true reason that helium dimer is not predicted to exist with a covalent bond. Of the four valence electrons in helium dimer, two will fill the bonding \( \sigma _{1s}\) molecular orbital, and the other two will fill the anti-bonding \( \sigma _{1s}^{\star }\) molecular orbital. The two electrons in the bonding \( \sigma _{1s} \) molecular orbital will achieve some stabilization relative to the \(1s\) atomic orbitals, but the two electrons in the anti-bonding \( \sigma _{1s}^{\star } \) molecular orbital will achieve greater de-stabilization relative to their position in the atomic orbitals. The net result is a less stable molecule than if the electrons remained in their respective \(1s\) atomic orbitals.
The electrons in antibonding orbitals cancel (and exceed) the stabilization resulting from electrons in bonding orbitals. Consequently, any system that has equal numbers of bonding and antibonding electrons will have a bond order of 0, and it is predicted to be unstable and therefore not to exist in nature (at least as a covalently bonding complex).
Based on molecular orbital theory discussed above, the \(\ce{He_2}\) molecule should not exist since no covalent bond formed between the helium atoms (Equation \ref{heliumdimer}). However, the molecular orbital description above neglects the van der Waals force that exists between the atoms as demonstrated by the existence of liquid helium (at 4 K). So a "molecule" composed of two helium atoms bound by the van der Waals force may exist by this attractive force instead - and it does.
A helium dimer molecule bound by Van der Waals forces was first proposed by John Clarke Slater in 1928 and observed in 1993 by Gentry and coworkers. Interestingly, \(\ce{He_2}\) is the largest known molecule of two atoms when in its ground state with an extremely long bond length with a separation of about 5,200 pm. The binding energy is only \(4.6 \times 10^{−5}\, kJ/mol\), so the \(\ce{He-He}\) bond is 5,000 times weaker than the covalent bond in the hydrogen molecule (Table 9.8.1 ).
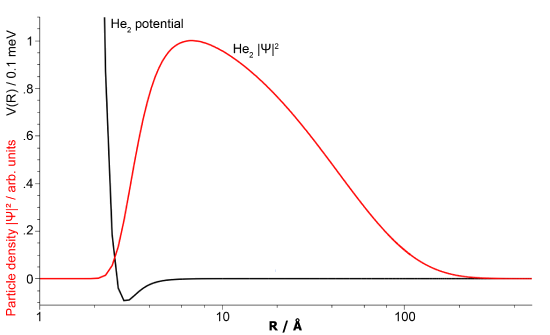
Conclusion
The decrease in energy caused by the bonding orbital (constructive interference of the atomic orbitals) is canceled by the increase in energy caused by the antibonding orbital (destructive interference of the atomic orbitals), so it is not energetically favorable for the helium atoms to be in such proximity, so if that situation arises, they'll separate quickly since there's no force keeping them there.

