10.2: Spin traps
- Page ID
- 370952
Many radicals are very reactive. This fact makes their detection during chemical reactions and in living cells very important, but it also makes their concentration very low, since often their formation reaction is slower than the reactions that destroy them again. For instance, concentration of the hydroxyl radical \({ }^{\bullet} \mathrm{OH}\), a reactive oxygen species (ROS) in living cells, is too low for EPR detection even under conditions where \({ }^{\bullet} \mathrm{OH}\) leads to cell damage or cell death. The situation is somewhat better for the superoxide anion radical \(\mathrm{O}_{2}^{2- \bullet}\), but physiologically relevant concentrations are hard to detect also for this species.
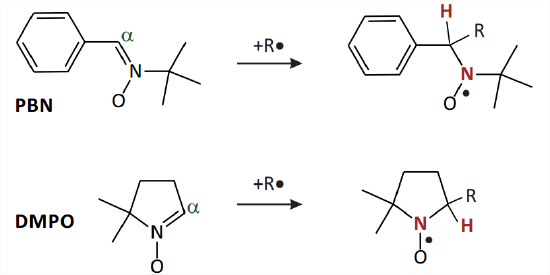
ROS and some other highly reactive radicals of interest are most easily detected by spin trapping. A spin trap (Fig. 10.8) is a diamagnetic compound that is primed to form a stable radical by reaction with an unstable radical. The most frequently used spin traps are nitrones that form nitroxide radicals by addition of the unstable radical to the \(\mathrm{C}\) atom in \(\alpha\) position of the nitrone group. The formed nitroxide radicals are not as stable as the ones used as spin labels, mainly because they contain a hydrogen atom in \(\alpha\) position to the \(\mathrm{N}-\mathrm{O}\) group. Their lifetime is usually on the minute time scale, which is sufficient for detection. The hyperfine coupling of the \(\mathrm{H}^{\alpha}\) atom is sensitive to the type of primary radical \(\mathrm{R}^{\bullet}\), i.e. to the nature of the other substituent at the \(C^{\alpha}\) atom. Furthermore, these nitrones are less sterically crowded than the ones that would yield more stable nitroxides and thus the nitrones are more reactive and trap radicals \(\mathrm{R}^{\bullet}\) more easily. In addition to the \(\mathrm{H}^{\alpha}\) hyperfine coupling, the hyperfine coupling of the \({ }^{14} \mathrm{~N}\) atom of the \(\mathrm{N}-\mathrm{O}^{\bullet}\) group is sensitive to the nature of \(\mathrm{R}^{\bullet}\). A database of experimental results supports assignment of \(R^{\bullet}\) in difficult cases: https://tools.niehs.nih.gov//stdb/index.cfm 1
1 Look a the "Hints for Using the Spin Trap Database" before you start your search. The keyword format is powerful, but not very intuitive.

