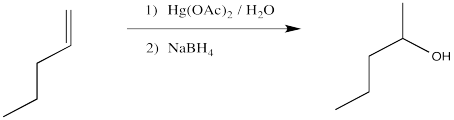EA5. Addition to Alkene Complexes
- Page ID
- 4286
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)EA5. Addition to Coordinated Alkenes
Alkenes can coordinate to transition metals to form alkene complexes. In some cases, coordination of the alkene to a metal leaves it susceptible to reaction with a nucleophile such as water.
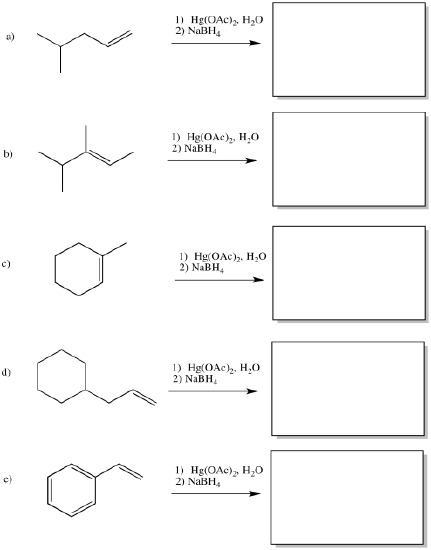
The classic case of nucleophilic donation to a coordinated alkene occurs with mercury (II) salts such as mercuric chloride, HgCl2, or mercuric acetate, Hg(OAc)2. The reaction, or rather the sequence of reactions, is called oxymercuration - demercuration or oxymercuration - reduction.
Problem EA5.1.
Compare the product of the reaction above to that obtained from treatment of 1-pentene with aqueous sulfuric acid.
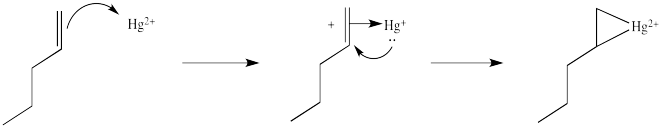
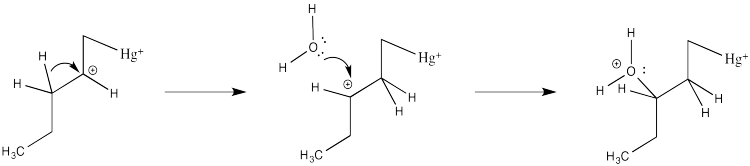
We will break the two different reactions in this sequence apart and focus only on the first one: oxymercuration. This reaction qualifies as an electrophilic addition because, as in the previous cases, it begins with donation of a π-bonding pair to an electrophile. In this case, we will consider the electrophile to be aqueous Hg2+ ion.
That electrophilic addition (from the alkene's perspective) results in the formation of an alkene complex. In reality, the mercury ion is also coordinated by several water molecules, but we will ignore them for simplicity.
You may know that alkene complexes are not observed with d0 transition metals. Although π-to-metal donation is the key event in the formation of such complexes, the alkene is just a little more sticky if the metal has d electrons. These electrons are able to "back-donate" into the alkene portion of the complex, adding extra stability to the interaction.
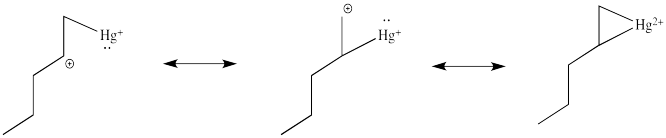
This situation is something like formation of a cyclic bromonium ion.
Note that the overall transfer of electrons is still from alkene to metal. That imbalance isn't apparent in a Lewis structure sense, in which case you can draw the structure so that there appears to be an equal trade. In a computational chemistry approach, in which we rely on basic principles of quantum mechanics and let computers churn out high-level calculations, we would still predict a little bit of positive charge on the alkene.
In the structure below, we have over-emphasized that charge, just to see what happens next in the reaction. When we draw it that way, it looks a lot more like simple addition of electrophile, such as H+, to alkene. We know it's more subtle than that. We'll get back to the real mechanism after a small detour.
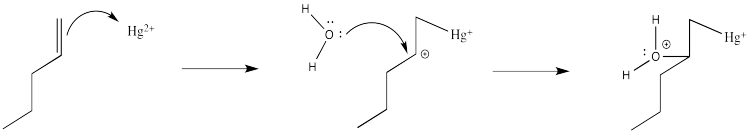
Of course, the next step is donation of a lone pair from a nucleophile to the almost-cationic carbon.
That looks easy. After that, deprotonation would result in the formation of a hydroxy group.
Problem EA5.2.
Suppose deprotonation is carried out by the acetate ion in solution. Draw a mechanism for this step.
How do we know the reaction doesn't happen through this simple cation?
Partly we know that because we know about alkene complexes. There are thousands of examples, structurally characterized by NMR spectroscopy and X-ray crystallography. In addition, we know it isn't a simple cation because nothing like the following scenario plays out during oxymercuration.
There are no hydride shifts. The cation stays put. The hydroxy group forms right where the alkene used to be. That means there is not a full carbocation like the one shown above.
If there isn't a real carbocation, though, why does the nucleophile end up at one particular end of the alkene? The hydroxy does end up at the position that would form the more stable cation. ( In other words, this reaction results in what is called "Markovnikov addition".)
There are a couple of reasons that could play a role. Foremost, the alkene isn't bound symmetrically. One end is held a little closer to the mercury than the other. Mostly that's because of sterics. Any other ligands on the mercury (such as those water molecules) push that more crowded end away a little bit. That slight asymmetry allows a little more charge to build on the more substitutted end of the alkene, which is therefore more electrophilic.
The final part of the reaction sequence is displacement of mercury from the hydroxyalkylmercury complex, effected through addition of sodium borohydride. The details of the reaction are usually dismissed in textbooks because they have little to do with electrophilic addition, the topic we are focusing on. However, the result is that the mercury is replaced by a hydrogen atom. The metal is converted to silvery, liquid, elemental mercury.
Problem EA5.3.
Suppose the demercuration reaction takes place via addition of hydride nucleophile to mercury, followed by reductive elimination. Draw this mechanism.
Problem EA5.4.
When mercuration takes place in a ethanol instead of water, an ether product results rather than an alcohol. Work through the mechanism and show the result of mercuration-demercuration in ethanol.