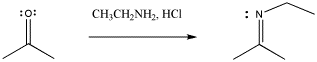CO2. General Reactivity Patterns
- Page ID
- 4228
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
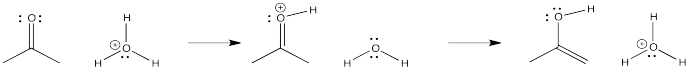
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the following pictures, a number of anions are added to a simple carbonyl compound, a ketone (2-propanone, or acetone). In each case, addition of the nucleophile is followed by addition of a proton source. Note that, overall, the reaction involves addition of the nucleophile to the carbonyl carbon and addition of the proton to the carbonyl oxygen.
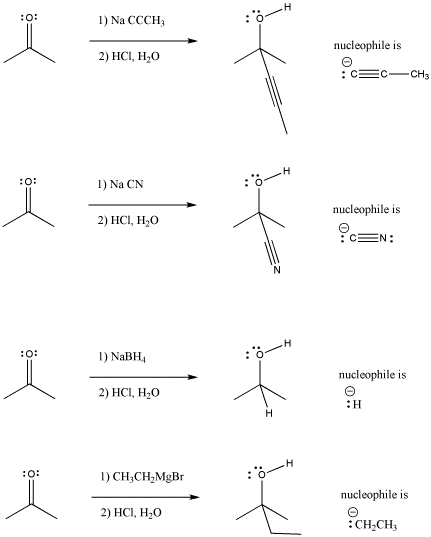
Figure CO2.1. Addition of some anionic (and "semi-anionic") nucleophiles to a ketone.
- Addition of anionic nucleophiles to ketones or aldehydes transforms the carbonyl into an alcohol.
Look at the way the reaction is presented in each case. The organic (carbon-based) starting material is presented on the left hand side of the reaction arrow. The reagent added to this starting material is often shown over the arrow. This reagent transforms the starting material into something else. That something else, the product, is shown to the right of the arrow.
Very often, the solvent for the reaction is shown underneath the arrow. The solvent is the liquid that is used to dissolve the starting material and reagents. This is done for a number of reasons. First, reactions generally happen much more quickly in solution than they do without a solvent. When dissolved, the reactants can move around more easily and bump into each other, as if they are swimming. Also, most useful reactions generate heat, and the solvent acts as a heat sink, carrying the excess heat away. (People who have not thought about the importance of solvent sometimes accidentally start fires as a result.) However, there are exceptions, and not all reactions need solvent.
These reactions shown above do need solvent, but the solvent is not shown for other reasons. These reagents must be added in a particular order: first the nucleophile and then the base. The nucleophile and base cannot be allowed to mix before the nucleophile has a chance to react with the carbonyl.
Problem CO2.1.
- For each of the cases shown above, use curved arrows to show the movement of electrons in the reaction between the anion and the carbonyl.
- Show the intermediate that results.
- Use curved arrows to show the movement of electrons in the reaction of the intermediate with acid to form the product.
Problem CO2.2.
- For each of the cases shown above, used curved arrows to show what would happen if acid were mixed with the nucleophile.
- Why would the nucleophile no longer be able to react with the carbonyl?
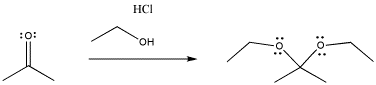
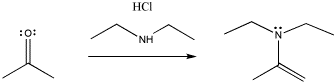
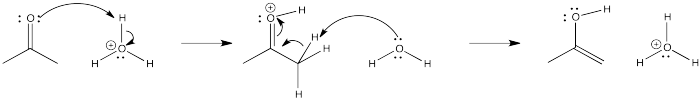
The pattern of reactivity is very different with another class of nucleophile. These could be called neutral nucleophiles (as opposed to anionic ones).
Take another look at the general pattern of reactivity for the anionic nucleophiles and the neutral nucleophiles. In the case of the anionic nucleophiles, the pattern is relatively easy to discover. The product has incorporated the nucleophile into its structure (or at least the anionic part of the nucleophile, which you will soon learn about). The nucleophile has attached at the carbonyl carbon. The carbonyl oxygen has become part of a hydroxyl group. These are very common patterns in the addition of nucleophiles to carbonyls.
In the case of the neutral nucleophiles, there are some similarities and some differences. The nucleophile is still incorporated into the product structure. It has added at the carbonyl position in the electrophile. However, the fate of the carbonyl oxygen is a little bit different with neutral nucleophiles. Generally, this atom is lost as a water molecule in these cases. If you look closely, you will be able to tell where the two protons come from in each case in order to form the water molecule. It's not really the HCl, which is only added in very tiny amounts and acts catalytically. The protons come from other positions in the nucleophile, and sometimes from the electrophile, too.
This chapter will help you to develop skills so that you can recognize where nucleophilic additions have taken place in reactions. You will also be able to predict what products may result from a nucleophilic addition.
Much of the chapter will focus on mechanisms of reaction. A reaction mechanism is, at the very least, the series of elementary steps needed to accomplish an overall reaction, and all of the intermediate structures that would be formed on the way from the reactants to the products.
- A reaction mechanism shows the structures of intermediates that occur after each elementary step.
An elementary reaction is typically a bond-forming or a bond-breaking step. In a bond-forming step, a pair of electrons are donated from one atom to another. In a bond-breaking step, a pair of electrons that were shared between two atoms are drawn to one end of the bond or the other, so that the bond breaks and the electrons end up on one atom only.
Very often, curved arrows are used to show the path that electrons take in these elementary steps. These arrows are always drawn from the source of the electrons to the place to which the electrons are attracted. These arrows help to illustrate bond-making and bond-breaking steps and also serve a book-keeping function, helping us to keep track of electrons over the course of the reaction.
- Curved arrows from the nucleophile to the electrophile show the path of electrons in the reaction.
Often, only one arrow is required in showing an elementary step, but not always. Sometimes, a bond-making step can happen at the same time as a bond-breaking step. This usually happens when an atom isn't large enough to accommodate the electrons from the new bond and sill keep the electrons from an old bond. In this case, two pairs of electrons move in the same elementary step, so two curved arrows are shown. Very rareley, more than two curved arrows are needed to show the events in one elementary step.
Sometimes other information is displayed in a reaction mechanism. Computational chemists will often leave out the curved arrow notation but will instead indicate the relative energy differences between all the intermediate structures along the reaction pathway. These energies may be experimentally determined (i.e. they may be based on the measurement of real reactions) or they may be calculated using an appropriate level of quantum theory. The energies may be displayed numerically, possibly in a table, or they may be illustrated using a picture, such as a reaction profile.