Solubility - What dissolves in What?
- Page ID
- 414161
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Solutions with Liquid Solvents
A solution is a homogeneous mixture in which the solute particles are so small that they cannot be filtered out and they do not scatter light. For a solution to form, there needs to be the correct combination of enthalpic (intermolecular forces) and entropic effects to enable the solvent and solute particles to interact strongly with each other without decreasing the entropy of the system. In this section we will closely examine the effects of enthalpy, but we will not spend too much time discussing the effects of entropy. To understand the effects of intermolecular forces, we need to go all the way back to bonds.
How Does Bond Polarity Affect/Determine Bond Type?
A bond is defined as the sharing of electrons by two atoms. Bond polarity occurs when two atoms share electrons unequally because of a difference in the strength of attraction for the shared electrons by the nuclei of the two different atoms. The strength of attraction scale that is most commonly used is electronegativity (EN). There are three common categories of bonds, based on the difference in electronegativity (∆EN) values of the two bonding atoms:
- If two atoms have identical, or similar EN values, their nuclei both pull on the shared electrons nearly equally. This equal sharing is called a nonpolar covalent bond – neither nucleus has a greater electron density after forming the bond than it did before forming the bond. Examples: H-H, F-F
- If two atoms have a modest difference in EN values, the nucleus of the atom with the higher EN value has a greater pull on the shared electrons, so that these electrons are more likely to be found closer to that nucleus than the nucleus with the lower EN value. This unequal sharing is called a polar covalent bond – there is a partial negative charge around the nucleus of the atom with the higher EN value because it is “using/borrowing” the electron(s) of the atom with the lower EN value. This leaves a partial positive charge around the nucleus of the atom with the lower EN value because its electron(s) are less likely to be found near it. Examples: H-Cl, C-F
- If two atoms have a large difference in EN values, the shared electrons are much more likely to be found closer to the nucleus of the atom with the higher EN value. There is always some small likelihood of finding the shared electron(s) near the nucleus of the atom with the smaller EN value, so there are never really separate, fully-charged ions in a sample of a pure substance. Nevertheless, it is common practice to designate these bonds as ionic bonds. This practice is reasonable because of the formation of separate, fully-charged ions when compounds involving these extremely polar bonds are placed in water. (See ion-dipole IMFs below.) Examples: Na-Cl, K-F
There are many approaches to the task of determining into which category a given bond should be placed. Some methods are more rigorous than others, but often the rigorous methods are too complicated to be used quickly and simply. You should find a method that you like and that serves your needs sufficiently and stick with it until you are asked to use a more rigorous method. The simplest method is that a bond between a metal and a nonmetal is ionic, whereas a bond between two nonmetals is covalent.
There are two common conventions used to show the polarity of a bond:
- lower case deltas (δ+ and δ-): These two symbols must always be used together, because they show the partial positive pole and the partial negative pole of the polar bond. Example:
δ+ δ-
B – Cl
- a dipole arrow
 : This symbol is used to show the positive end of the dipole and the negative end at which the electron density is greatest. Example:
: This symbol is used to show the positive end of the dipole and the negative end at which the electron density is greatest. Example:
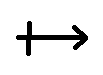
B – Cl
What is Molecular Polarity?
The concept of molecular polarity is most easily applied to small molecules, but it can be applied to larger molecules with the understanding that larger molecules may have multiple “identities.” The first task in determining molecular polarity is to identify whether a substance is comprised of ions or of molecules. Individual small ions are not polar, because they do not have a charge separation that leads to the existence of a positive pole and a negative pole. Thus, if you identify a compound as consisting of ions (either monatomic or small polyatomic), you are no longer concerned about molecular polarity of that substance. To determine the molecular polarity of a compound that contains only covalent bonds, you need to consider three aspects of the molecule:
- the shape of the molecule
- the polarity of all of the bonds in the molecule
- the presence of atoms with lone pairs.
Taking these three aspects into account, you should be able to determine if a small molecule is polar or nonpolar. Some helpful shortcuts that are almost always true:
a) If the central atom(s) in a molecule has at least one lone electron pair, the molecule will be polar.
NH3 is polar, and so is O3
b) If the molecule contains only nonpolar bonds and there are no lone pairs on the central atom(s), the molecule will be nonpolar.
All CxHy molecules are nonpolar
c) If all of the atoms attached to the central atom are of the same element and there are no lone pairs on the central atom, the molecule is nonpolar.
CCl4 and CO2 are nonpolar
d) If the atoms attached to the central atom are of different elements, the molecule is polar.
CH2Cl2 and OCS are polar
e) If a molecule of a compound contains at least one N, O, F, or Cl atom, the molecule is usually polar, unless c) is true.
CH3CH2OH, HF, CH3NH2, and CH3Cl are all polar
f) When molecules get large enough, they might have polar sections and nonpolar sections. A general rule of thumb for carbon-containing compounds is that molecules with 4/5 or fewer C atoms per polar bond can be considered to act as if they are polar. Thus, molecules with 4/5 or more C atoms per polar bond can be considered to act as if they were nonpolar.
CH3CH2OH is considered polar; CH3CH2CH2CH2CH2CH2CH2OH is considered nonpolar; CH3CH2CH2CH2OH is right on the polar/nonpolar divide
g) All single atoms are nonpolar. Most, but not all, molecules of elements are nonpolar.
Polar molecules have a dipole moment greater than zero, and thus are considered dipoles.
What is an Intermolecular Force?
If you have a collection of independent atoms, molecules, or ions, these particles will always exert forces of attraction and repulsion among themselves as they more closely approach each other. The repulsive forces tend to be ignored, but they do exist and come about mainly because of the repulsion of the negatively-charged electron clouds surrounding each particle (even for cations, although the positive charges do lead to repulsion with other cations and the nucleus of other atoms.)
A great deal of time is spent studying the attractive forces among independent particles. These forces are what enable molecules and atoms to gather together and remain in a condensed phase as liquids and solids. Collectively, these attractive forces are commonly known as intermolecular forces (IMFs). They are the forces of electrostatic attraction that exist between separate particles.
How is an IMF different from a bond?
Along with the common definition that bonds occur within molecules and IMFs occur between molecules, there are two closely related factors that distinguish bonds and IMFs:
- The atomic nuclei are closer together in a bond between two atoms than in an IMF between two particles.
- The potential energy released when a bond forms is greater than the potential energy released when an IMF forms.
These statements are generalizations, and there are certainly exceptions, but they hold true for most common substances.
What are the Types of IMFs?
The simplest way to categorize IMFs is to classify the particles that are interacting as one of the following:
- ions
- polar molecules
- nonpolar molecules
From these three particle types, you can describe five different types of interparticle interactions:
- dipole/dipole forces
- ion/dipole forces
- dipole/induced dipole forces.
- ion/induced dipole forces
- dispersion or London forces
(All particles exert and experience London forces, but the only forces among nonpolar particles are London forces)
The only remaining interaction would be ions interacting with ions, but that is an ionic bond. There is, however, a sixth IMF. It is a case of extreme dipole/dipole interactions:
- molecules with very polar bonds interacting with molecules with very polar bonds – known as hydrogen bonding (but it is NOT a bond!)
The only bonds that are polar enough to result in a large enough partial positive charge on a small atom are:
δ- δ+ δ- δ+ δ- δ+
F—H, O—H, and N—H bonds.
The only atoms that have a large enough EN value to result in a large enough partial negative charge on a small atom are:
δ- δ- δ-
F, O, and N
What Substances Will Mix with Each Other to Create a Solution?
This discussion ignores entropy and focuses on enthalpy, but entropy definitely plays a role. That discussion is for later.
A solution is a homogeneous mixture in which the solute particles are so small that they cannot be filtered out and so dispersed throughout the mixture that they do not scatter a beam of light that travels through the mixture. The probability of one substance dissolving/mixing in/with another substance to form a solution depends on whether or not the newly formed IMFs are as energetically favorable as the currently existing IMFs. Thus, the mutual solubility of two substances depends mainly on the substance with the IMFs that involve the largest charge separation. In other words, there is a hierarchy of what will mix:
a) Ionically-bonded solids will form a solution (dissolve) in a solvent only if the solvent can provide very strong ion/dipole interactions.
- Usually water is the only material that can dissolve inorganic salts to any great extent, although some inorganic salts dissolve slightly in methanol and DMSO. You can find tables of solubility rules for common inorganic salts in water. One of these rules is that almost all compounds containing Group IA cations are soluble.
- Organic salts have a reasonable solubility in many polar organic solvents, but are generally much more soluble in water than in these organic solvents. The non-organic counter cations are usually Na+, K+, or NH4+, all of which generally form soluble salts. The non-organic counter anions are usually Cl-, NO3-, and SO42-, all of which generally form soluble salts.
b) Water will form a solution (mix or dissolve) with another covalently-bonded substance only if:
i) the molecules of that substance can form hydrogen bonds with the water molecules.
-The molecules of the other substance do not need to be able to form hydrogen bonds among themselves when in the pure form, but they must be polar molecules and contain an N, O, or F atom that has a partial negative charge. Acetone is an example of such a substance.
The ability of a molecule to form hydrogen bonds with water molecules does not guarantee that the molecule will dissolve in water. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen bonds among themselves. Thus, the larger the nonpolar section, the less soluble the substance is in water. A general rule of thumb is the 4-5 carbon atom rule: Molecules with fewer than 4-5 C atoms per hydrogen bonding group will likely be soluble in water. Molecules with more than 4-5 C atoms per hydrogen bonding group will likely be insoluble in water. The water solubility of molecules with 4-5 C atoms per hydrogen bonding group is best determined by experiment or by a search of the literature. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four C atoms per molecule: butanoic acid is soluble with water in all ratios; 1-butanol is reasonably soluble in water; and diethyl ether is so slightly soluble in water that it is considered insoluble.
or
ii) the molecules of that substance react with water molecules to form ions, which then dissolve in the water because of ion/dipole interactions.
- The six strong acids (HCl, HBr, HI, HNO3, HClO4, and H2SO4) are the most common examples. All of these acids have Ka values greater than 10. (Remember, the Ka value tells you how likely it is for the acid to react with water, and a Ka greater than 1 means that the reaction is product-favored at equilibrium)
All molecular weak acids and molecular weak bases react with water to some extent to create ions, but these weak acids all have Ka values that are less than 1 and these weak bases all have Kb values that are less than 1. Thus, all these reactions are considered reactant-favored, and for purposes of predicting water solubility, we ignore the fact that some ions are formed. Remember that the intact weak acid or weak base molecules may themselves dissolve in water through hydrogen bonding. (See section b) (i) above.
c) The common adage “Like dissolves like” works well for most organic compounds. Polar compounds tend to mix well with other polar compounds. The IMFs involved can be hydrogen boding, dipole/dipole, and London forces. Nonpolar compounds tend to mix well with other nonpolar compounds, with London forces being the only IMF involved.
- With a few notable exceptions (CCl4, for instance), organic compounds containing chlorine and bromine and iodine do have a dipole moment, and are therefore polar molecules. These molecules cannot, however, form hydrogen bonds with water, nor are the halogen atoms present as ions. Thus, halogenated organic compounds do not dissolve well in water.
- Because most organic compounds have at least one small nonpolar section on their molecules, some polar organic compounds mix well with nonpolar organic compounds.
d) Solubility in aqueous acid solutions and/or aqueous base solutions is a confusing concept, mainly because of the way that chemists talk about the process. The statement, “Check the solubility of benzoic acid in 3 M NaOH” may be clear to a practicing chemist, but it is misleading to most beginning students. The statement would be much clearer if it were written, “Check the water solubility of the organic anion formed when benzoic acid reacts with the OH- ion in an aqueous 3M NaOH solution.” The same clarity, or lack thereof, is true for statements about solubility in aqueous acid solutions.
The order of events when an aqueous solution of a base is added to an organic compound that can act as an acid is:
i) The organic compound reacts with the OH- ion, donating an H+ ion to the OH- ion.
ii) The products of this reaction are water and the conjugate base of the organic compound. If the original organic compound was a neutral molecule, the conjugate base will be a water-soluble anion.
The order of events when an aqueous solution of an acid is added to an organic compound that can act as a base is:
i) The organic compound reacts with the acid, accepting an H+ ion.
ii) The products of this reaction are the conjugate base of the acid, and the conjugate acid of the organic compound. If the original organic compound was a neutral molecule, the conjugate acid will be a water-soluble cation.
If no acid/base reaction occurs between the aqueous acid/base and the organic compound, then the organic compound remains as a neutral substance, and maintains its original solubility (or lack thereof ) in water.
If knowing the solubility of two compounds is an essential piece of information for your work, it is always best to not only look up the solubility data, but also perform careful solubility tests yourself in the lab.

