Properties of Esters
- Page ID
- 3909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Esters are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an ester the hydrogen in this group is replaced by a hydrocarbon group. This could be an alkyl group like methyl or ethyl, or one containing a benzene ring such as a phenyl or benzyl group. The most commonly discussed ester is ethyl ethanoate. In this case, the hydrogen in the -COOH group has been replaced by an ethyl group. The formula for ethyl ethanoate is:
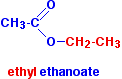
Notice that the ester is named the opposite way around from the way the formula is written. The "ethanoate" bit comes from ethanoic acid. The "ethyl" bit comes from the ethyl group replacing the hydrogen.
In each case, be sure that you can see how the names and formulae relate to each other.
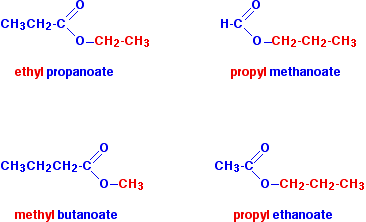
Notice that the acid is named by counting up the total number of carbon atoms in the chain - including the carbonyl carbon. So, for example, \(CH_3CH_2COOH\) is propanoic acid, and \(CH_3CH_2COO\) is the propanoate group.
Fats and oils
Animal and vegetable fats and oils are composed of long-chain, complicated esters. The physical differences observed between a fat (like butter) and an oil (like sunflower oil) are due to differences in melting points of the mixture of esters they contain. If the melting point of the substance is below room temperature, it will be a liquid - an oil. If the melting point is above room temperature, it will be a solid - a fat. The causes of the differences in melting points are discussed below.
Fats and oils as big esters
Esters can be made from carboxylic acids and alcohols. This is discussed in detail on another page; in general terms, the two combine together, losing a molecule of water in the process. Consider a very simple ester such as ethyl ethanoate. The figure below shows its formation from ethanoic acid and ethanol.

Figure: The diagram shows the relationship between the ethanoic acid, the ethanol and the ester. This is not intended to be a full equation. Water, of course, is also produced.
The same process can be carried out for more complicated alcohols. The diagram below shows the structure of propane-1,2,3-triol (commonly known as glycerol).
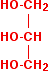
Just as with the ethanol in the previous equation, I've drawn this back-to-front to make the next diagrams clearer. Normally, it is drawn with the -OH groups on the right-hand side. By the esterification process shown above, three ethanoate groups can be formed.
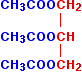
Lengthening each carbon chain creates a triglyceride, otherwise known as a fat.
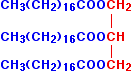
The acid CH3(CH2)16COOH is called octadecanoic acid, frequently referred to by its common name, stearic acid. The full name for the ester of this with propane-1,2,3-triol is propane-1,2,3-triyl trioctadecanoate, unsurprisingly known by by its common name of glyceryl tristearate.
Saturated and unsaturated fats and oils
If the fat or oil is saturated, the acid from which it is derived has no carbon-carbon double bonds in its chain. Stearic acid is a saturated acid; therefore glyceryl tristearate is a saturated fat. If the acid has just one carbon-carbon double bond somewhere in the chain, it is called mono-unsaturated. If it has more than one carbon-carbon double bond, it is polyunsaturated.
The acids below are saturated acids, and will therefore form saturated fats and oils:

Oleic acid is a typical mono-unsaturated acid:
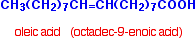
Linoleic and linolenic acids are typical polyunsaturated acids.
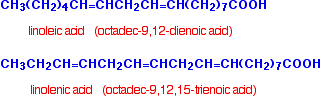
The terms "omega-6" and "omega-3" have become popular in the context of fats and oils. Linoleic acid is an omega-6 acid. This indicates that the first carbon-carbon double bond starts on the sixth carbon from the CH3 end. Linolenic acid is an omega-3 acid for the same reason. Because of their relationship with fats and oils, all of the acids above are sometimes described as fatty acids.
Physical properties
Boiling points
Small esters have boiling points which are similar to those of aldehydes and ketones with the same number of carbon atoms. Esters, like aldehydes and ketones, are polar molecules and so have dipole-dipole interactions as well as van der Waals dispersion forces. However, they do not form ester-ester hydrogen bonds, so their boiling points are significantly lower than those of an acid with the same number of carbon atoms.
| molecule | type | boiling point (°C) |
|---|---|---|
| CH3COOCH2CH3 | ester | 77.1 |
| CH3CH2CH2COOH | carboxylic acid | 164 |
Solubility in water
Small esters are fairly soluble in water but solubility decreases with increasing chain length, as shown below:
| ester | formula | solubility (g per 100 g of water) |
|---|---|---|
| ethyl methanoate | HCOOCH2CH3 | 10.5 |
| ethyl ethanoate | CH3COOCH2CH3 | 8.7 |
| ethyl propanoate | CH3CH2COOCH2CH3 | 1.7 |
The reason for this trend in solubility is that although esters cannot hydrogen bond with each other, they can hydrogen bond with water molecules. One of the partially-positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on one of the oxygen atoms in an ester, forming a hydrogen bond. Dispersion forces and dipole-dipole attractions are also present.
Forming these intermolecular attractions releases some of the energy needed to solvate the ester. As chain length increases, the hydrocarbon portion forces itself between water molecules, breaking the relatively strong hydrogen bonds between water molecules without offering an energetic compensation; furthermore, the water molecules are forced into an ordered alignment along the chain, decreasing the entropy in the system. This makes the process thermodynamically less favorable, and so solubility decreases.
The physical properties of fats and oils
Solubility in water
Fats and oils are not water soluble. The chain lengths are so great that far too many hydrogen bonds between water molecules must be broken; this is not an energetically profitable arrangement.
Melting point
Melting points determine whether the substance is a fat (a solid at room temperature) or an oil (a liquid at room temperature). Fats normally contain saturated chains. These allow more effective van der Waals dispersion forces between the molecules: more energy is required to separate the chains, increasing the melting point.
A greater number of double bonds, or degree of unsaturation, in the molecules results in a lower melting point, because the van der Waals forces are less effective. The efficacy of van derWaals forces depends on the ability of molecules to pack closely together. The presence of carbon-carbon double bonds in the chains disrupts otherwise tidy packing. Here is a simplified diagram of a saturated fat:

The hydrocarbon chains are, of course, in constant motion in the liquid, but it is possible for them to lie tidily when the substance solidifies. If the chains in one molecule can lie tidily, that means that neighboring molecules can get close. That increases the attractions between one molecule and its neighbors and so increases the melting point.
Unsaturated fats and oils have at least one carbon-carbon double bond in at least one chain. Rotation about a carbon-carbon double bond is constrained, locking a permanent kink into the chain. This makes packing molecules close together more difficult. If the chains cannot pack well, the van der Waals forces will be less effective. This effect is much stronger for molecules in which the hydrocarbon chains at either end of the double bond are arranged cis- to each other, as shown in the figure below:
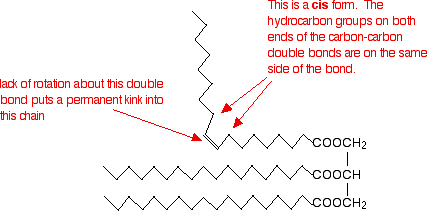
In the trans- conformation, the effect is not as marked. It is, however, rather more than the diagram below suggests because of the changes in bond angles around the double bond compared with the rest of the chain.
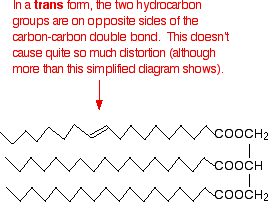
Trans-fats and oils have higher melting points than their corresponding cis- conformations because the packing is not interrupted to the same degree. Naturally occurring unsaturated fats and oils tend to adopt the cis- conformations.
Contributors
Jim Clark (Chemguide.co.uk)


