II. Azides
- Page ID
- 24543
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A. Reactions
1. Reduction
Azides are reduced to tin-substituted amines by reaction with tin hydrides.1–9 These tin-containing compounds generally are not isolated; rather, each is converted into either the corresponding amine or an amine derivative. Amine derivatives include compounds formed by reaction of a tin-containing product with an acid halide,1,2 anhydride,3 or ester.4 Free amines are liberated from tin-containing products by a hydrolysis that often takes place during chromatographic purification.5–9 Examples of reactions producing amine and amine derivatives are found in equations 17 and 2,1 respectively. Conversion of an azide into the corresponding amine also can take place when tri-n-butyltin hydride is replaced by tris(trimethylsilyl)silane.10
.png?revision=1&size=bestfit&width=410&height=175)
.png?revision=1&size=bestfit&width=390&height=149)
Treatment of azides with tri-n-butyltin hydride does not always cause reaction of the azido group.11–13 Carbohydrates containing O‑thiocarbonyl substituents (eq 3)11,12 or iodine atoms (eq 4)13 undergo chemoselective reaction that leaves the azido group intact. If the amount of Bu3SnH is sufficient and the conditions are conducive, azido groups will react after the replacement of more reactive groups is complete.13
.png?revision=1&size=bestfit&width=335&height=107)
.png?revision=1&size=bestfit&width=400&height=97)
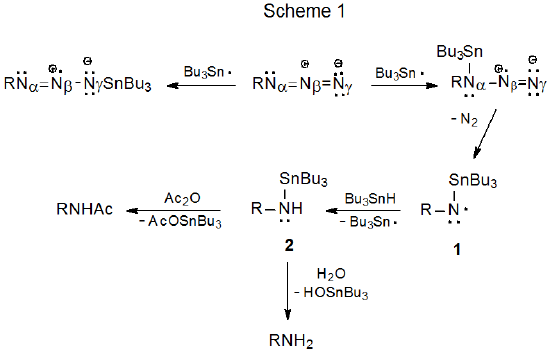
The tri-n-butyltin radical potentially can add to either Nα or Nγ in an azido group (Scheme 1), but since the intermediate radical 1 is thought to be a precursor to the tin-containing product 2, it is assumed that the initial addition of Bu3Sn· is to Nα.14–16 As mentioned above and indicated in Scheme 1, normal procedure calls for either hydrolysis or derivatization prior to product isolation.
2. Bromination
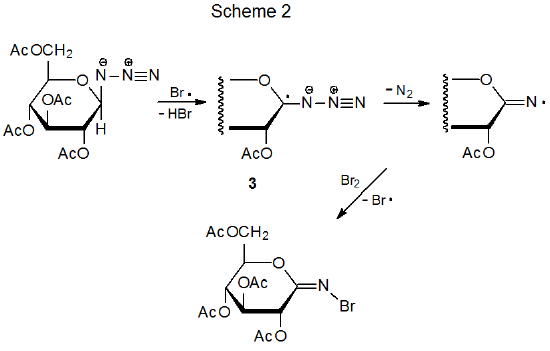
Free-radical bromination of a glycosyl azide produces the corresponding N-bromoglycosylimine.17,18 The reaction shown in Scheme 2 begins such a process when H-1 is abstracted regioselectively to give the radical 3, an intermediate that is stabilized by both the ring oxygen atom and the azido group. Loss of molecular nitrogen, followed by bromine-atom abstraction, completes the reaction.
3. Nitrile Formation
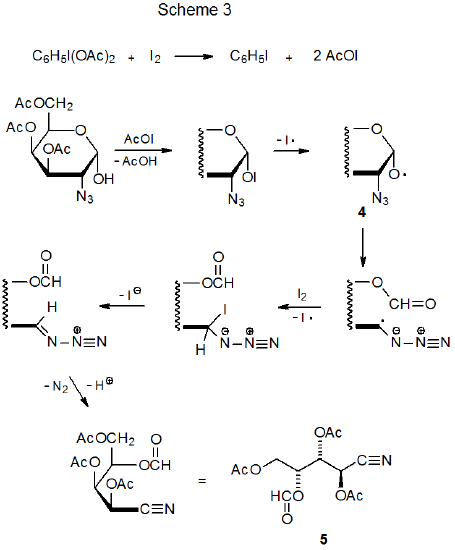
If an oxygen-centered radical and an azido group are attached to adjacent carbon atoms, the bond between the carbon atoms cleaves in a reaction leading to a nitrile.19 In the example shown in Scheme 3, the alkoxy radical 4 fragments to open a six-membered ring; a sequence of steps then leads to the nitrile 5. Similar ring opening occurs in compounds with five-membered rings.19
B. Synthesis
1. Carbohydrate Radical Addition to Ethanesulfonyl and Benzenesulfonyl Azides
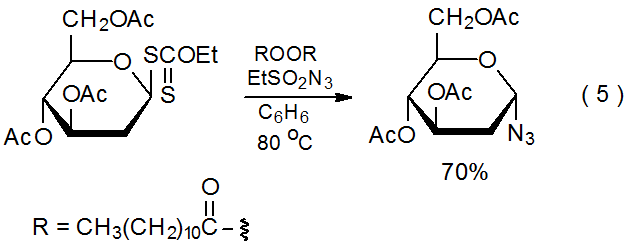
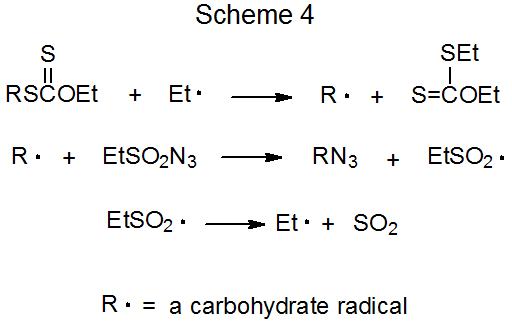
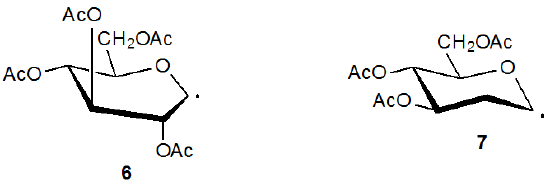
It is possible to synthesize a carbohydrate azide by reaction of the corresponding xanthate with ethanesulfonyl azide20 or benzenesulfonyl azide20,21 (eq 521). The propagation steps for this type of reaction are outlined in Scheme 4.21 As shown in eq 6, the presence of an acetoxy group at C-2 reduces product yield when compared to reaction in which such a group is absent (eq 5). The intermediate pyranos-1-yl radical 6, which adopts a B2,5 boat conformation, is more hindered at C-1 than the pyranos-1-yl radical 7, which lacks a C-2 substituent and has a chair conformation. (Section IV of Chapter 6 in Volume I contains more information about the conformation of pyranos-1-yl radicals.) In addition to being more hindered at C-1, the radical 6 is less nucleophilic due to the electron-withdrawing nature of the C-2 acetoxy group.
.png?revision=1&size=bestfit&width=320&height=127)
.png?revision=1&size=bestfit&width=320&height=131)
2. Azide Radical Addition to an Unsaturated Carbohydrate
a. Azide Radicals
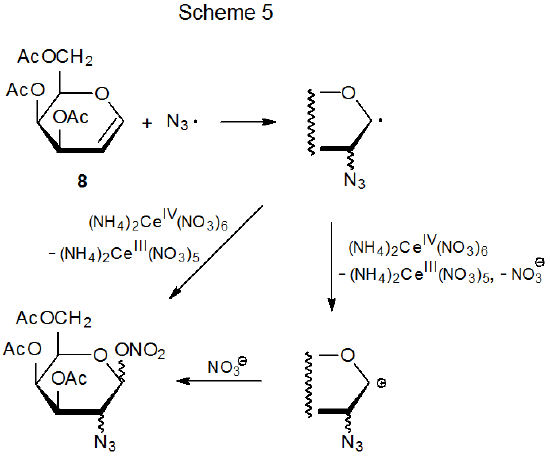
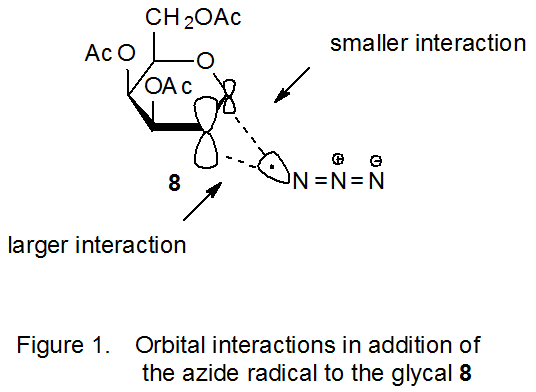
Azide ion is converted into azide radical by reaction with oxidizing agents that include ammonium cerium(IV) nitrate (eq 7)22 and (diacetoxyiodo)benzene (eq 8).23 The electrophilic nature of the azide radical is attested to by its addition to electron-rich double bonds such as those found in glycals.24–47 Atomic orbital coefficients can be critical in determining regioselectivity in a reaction with an early transition state because the rate constant for the bond-forming process between two atoms depends in its early stages on the magnitude of the coefficients of the interacting frontier orbitals.28,29 In the azide radical addition pictured in Scheme 5, the frontier orbitals are the SOMO of the azide radical and the HOMO of the D-galactal 8. The more reactive position for addition to the double bond in 8 is at C-2 because, based on simple model systems,30 the atomic orbital coefficient for the HOMO is larger at C-2 than at C‑1 (Figure 1).
.png?revision=1&size=bestfit&width=410&height=22)
.png?revision=1&size=bestfit&width=340&height=22)
b. Azidonitration
Azidonitration takes place when the azide radical is produced by oxidation of the azide ion by ammonium cerium(IV) nitrate in the presence of a glycal.24–27,31–39 The stereoselectivity of azide radical addition is dependent on glycal structure; thus, N3· adds in a highly selective fashion to the α face of the D-galactal 8 because the β face is well protected by ring substituents (eq 9).24 Less effective protection of the β face, which occurs when 8 is replaced by the D‑glucal 9, leads to stereoselectivity that varies as the reaction conditions change (eq 10).37 When a 4,6-O-isopropylidene group is incorporated into the D-glucal structure, it creates a compound that is conformationally less mobile. With this reduction in conformational mobility comes greater stereoselectivity in addition of the azide radical to the D-glucal derivative (eq 11).37
.png?revision=1&size=bestfit&width=380&height=269)
.png?revision=1&size=bestfit&width=420&height=150)
.png?revision=1&size=bestfit&width=420&height=119)
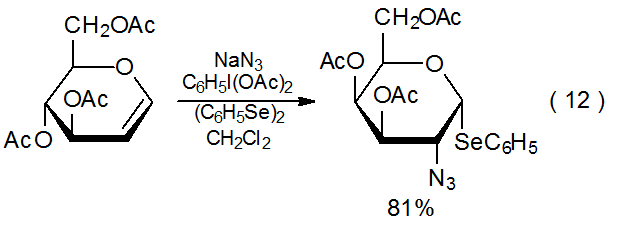
c. Azidophenylselenylation
When azide ion is oxidized by (diacetoxyiodo)benzene, the resulting azide radical will add to a glycal to produce a phenyl selenide if diphenyldiselenide is present in the reaction mixture (eq 1240).39–48 The normal solvent for this reaction is dichloromethane, but due to limited solubility of sodium azide in this solvent, reaction usually is heterogeneous, a situation that causes product yields to suffer except in quite dilute solutions. If, however, the azide radical is generated from trimethylsilyl azide, solutions are homogeneous and good product yields are realized (eq 13).49
.png?revision=1&size=bestfit&width=310&height=113)
.png?revision=1&size=bestfit&width=310&height=151)
d. Azidohalogenation
The azide radical also forms by photolysis of chloroazide (eq 14). When this reaction takes place in the presence of a glycal, azidochlorination results (eq 15).50 The stereoselectivity of glycal addition by the azide radical formed from photolysis is similar to that observed when this radical is generated by azide ion oxidation. The photochemical reaction is different, however, in that a small amount (13%) of addition occurs in which the positions of the azido and chloro substituents are interchanged. Since photolysis of chloroazide also produces the electrophilic chlorine atom (eq 14), it is reasonable to expect that sometimes a chlorine atom would add to a glycal at C-2.
.png?revision=1&size=bestfit&width=225&height=25)
.png?revision=1&size=bestfit&width=385&height=148)
3. Atom Replacement by an Azido Group
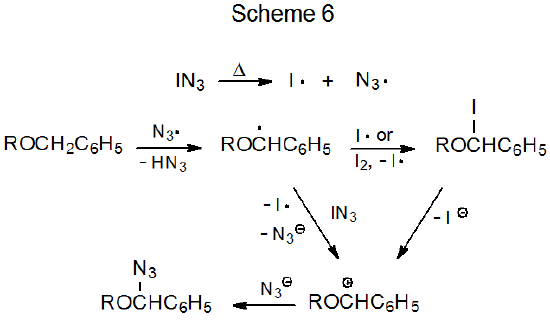
Reaction between iodoazide and a carbohydrate containing a benzyloxy group leads to hydrogen atom replacement by an azido group (eq 16).51 In this reaction the azide radical abstracts one of the reactive hydrogen atoms from the benzyloxy group to form a benzylic radical. The azido group then can be introduced by one of the proposed pathways shown in Scheme 6.
.png?revision=1&size=bestfit&width=345&height=121)