III. Photochemical Electron Transfer to Carboxylic Acid Esters
- Page ID
- 24043
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A. Acetates and Pivalates
Photochemical electron transfer from excited hexamethylphosphoramide (HMPA) to an O-acyl group in a carbohydrate begins a series of events that result in replacing each O-acyl group with a hydrogen atom. An example of a typical reaction is shown in eq 5.27 The temperature at which this reaction can be conducted (~25 oC) is synthetically far more attractive than the 140 oC needed for the corresponding thermal reaction of an acetylated carbohydrate (eq 3).23 Photochemical electron transfer to acetates has been used for the synthesis of a number of deoxy sugars.27–34 Esters of pivalic acid, which also can serve as substrates in this type of reaction,28,35–41 sometimes give better yields than the corresponding acetates.28,35,36
.png?revision=1&size=bestfit&width=335&height=104)
1. Reaction Mechanism
Photochemical electron transfer begins with absorption of light by HMPA to produce a highly reactive, electronically excited molecule that ejects an electron into the solution (Scheme 5).42.43 The ejected electron is captured by the acylated carbohydrate to produce a radical anion that cleaves to give a carboxylate anion and a carbohydrate radical (R·).43 Hydrogen-atom abstraction by the carbohydrate radical then completes the replacement process (Scheme 5). The water present in the reaction mixture extends the lifetime of the solvated electron and, in so doing, increases the probability that this electron will be captured by a molecule of ester.43 (The importance of water to the success of this process is demonstrated by the yields of the reactions shown in eq 6.44,45)
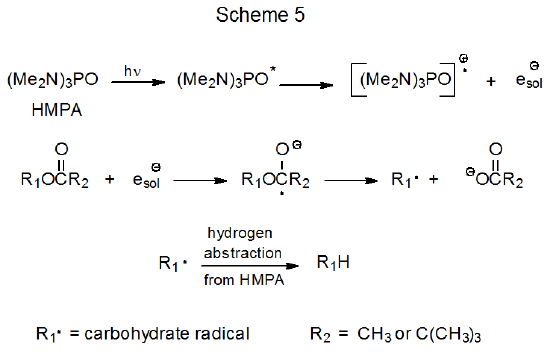
.png?revision=1&size=bestfit&width=355&height=164)
2. Alcohol Regeneration
Ester photolysis in aqueous HMPA sometimes regenerates the alcohol from which the ester was synthesized (eq 7).34 In some instances, alcohol formation may be due to nonphotochemical ester hydrolysis. Nonphotochemical reaction provides a reasonable explanation for the easily hydrolyzed, anomeric acetate shown in eq 8 undergoing only hydrolysis (no deoxygenation) when photolyzed in aqueous HMPA.34 Even though simple hydrolysis may be significant for some compounds, as described below, alcohol regeneration during photolysis of other, probably most, esters must occur in a different way.
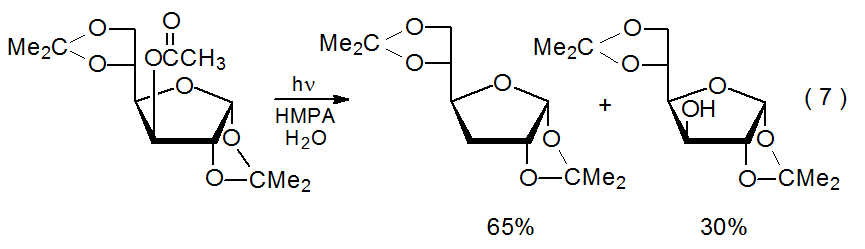.png?revision=1&size=bestfit&width=435&height=125)
.png?revision=1&size=bestfit&width=340&height=105)
Alcohol formation during ester photolysis cannot be explained, in general, by simple hydrolysis because, as is shown by the reaction pictured in Scheme 6, the yield of the alcohol can depend on the concentration of the starting ester.43 One explanation for this dependence begins with the ester 5 capturing a solvated electron to form the radical anion 6. This radical anion then abstracts a hydrogen atom from a second molecule of 5 to produce the anion 7, which then forms an alkoxide ion that protonates to give the observed alcohol.43 Since, according to this explanation, raising ester concentration should increase the rate of hydrogen-atom abstraction to give 7 but not the rate of the competing β-cleavage that forms R·, greater ester concentration should increase the amount of alcohol (ROH) produced at the expense of the deoxygenated product (RH).
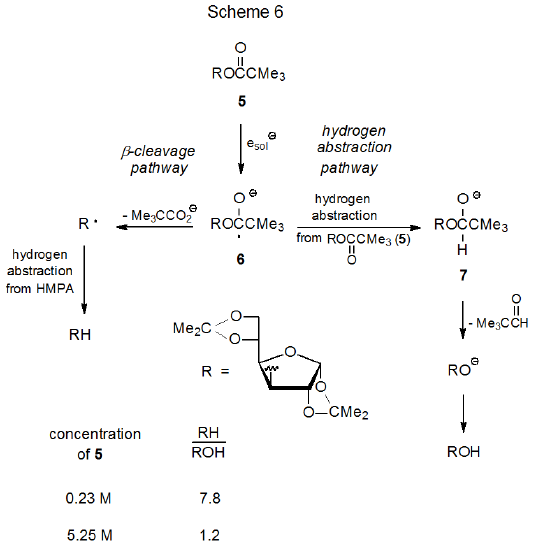
A critical question about the mechanism for alcohol formation presented in Scheme 6 concerns whether the radical anion 6 can abstract a hydrogen atom from the ester 5. The evidence found in eq 9 supports the idea that 5 can function as a hydrogen-atom donor. Irradiation of 5 in HMPA‑d18/D2O gives a 27% yield of 8, a reduction product that contains no deuterium. Since the only source for the second hydrogen atom at C-3 in 8 is one of the carbohydrates in the reaction mixture and since the ester 5 is the only carbohydrate present at the beginning of the reaction, abstraction from 5, at least in the early stages of reaction, seems unavoidable. If 5 can act as a hydrogen-atom donor in the formation of 8, it becomes a strong candidate for the same role in the conversion of the radical anion 6 into the alkoxide ion 7 (Scheme 6).
.png?revision=1&size=bestfit&width=435&height=140)
3. Competition From Light-Absorbing Chromophores
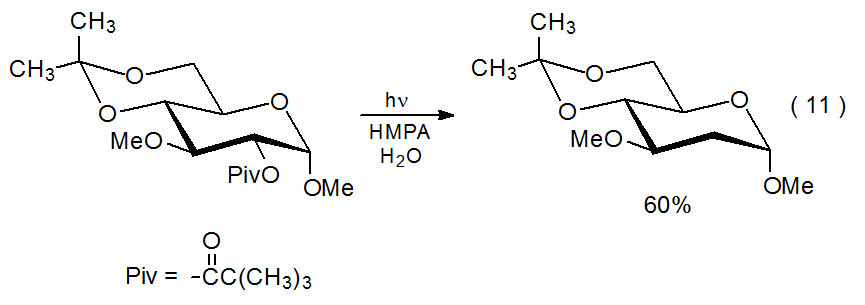
If an ester contains a strongly absorbing chromophore, HMPA excitation will be effectively precluded because most of the incident light will be absorbed by the ester. Failure to excite HMPA will forestall replacement of the acyloxy group with a hydrogen atom by preventing electron transfer. The light absorbing properties of the benzoyloxy group, for example, render benzoates much less desirable participants in these electron-transfer reactions because far less incident light reaches the HMPA. This means that an important factor in reaction of simple acetates and pivalates is that these compounds contain no strongly absorbing chromophore.42 An example of an ester that fails to undergo replacement of the acyloxy group due to the presence of a light-absorbing substituent (i.e., the 4,6-O-benzylidene group) is shown in eq 10.41 Even though the benzylidene group is removed during photolysis, the aromatic chromophore remains in the solution and continues to absorb the incident light. Changing 4,6-O-benzylidene to 4,6-O-isopropylidene protection allows reaction to proceed in the normal fashion (eq 11).41
.png?revision=1&size=bestfit&width=400&height=134)
.png?revision=1&size=bestfit&width=435&height=153)
B. m-(Trifluoromethyl)benzoates
A m-(trifluoromethyl)benzoate will accept an electron from excited N‑methylcarbazole (11) in a reaction that leads to replacement of the acyloxy group with a hydrogen atom; for example, photolysis of 2',3',5'-tri-O-[m-(trifluoromethyl)benzoyl]-adenosine (9) produces the 2',3'-dideoxyadenosine derivative 10 (eq 12).46 (Most,46–52 but not all,48 compounds reported to undergo this type of reaction are nucleosides.) Reactions, such as the one shown in eq 12, are regioselective because the radical anion generated from a m-(trifluoromethyl)benzoyl group does not fragment to give a primary radical.
.png?revision=1&size=bestfit&width=410&height=265)
Photochemical electron transfer involving m-(trifluoromethyl)benzoates and N-methylcarbazole (11) has several advantages over electron transfer between HMPA and acetates or pivalates. One of these is that N‑methylcarbazole has greater molar absorptivity than HMPA, a fact that renders the carbohydrate reactant less likely to stop the reaction by absorbing the incident light.52 From a safety point of view, eliminating HMPA from the reaction mixture avoids handling a highly toxic, cancer-suspect agent. Because the m‑(trifluoromethyl)benzoyl group is an effective electron acceptor (better than an acetyl or pivaloyl group) few substituents in the carbohydrate will compete with this group for an electron donated by excited N-methylcarbazole (11); consequently, reactions of m-(trifluoromethyl)benzoates usually are highly chemoselective. An example of this selectivity is shown in eq 13, where the benzoyl group remains bonded to C-3' while the m‑(trifluoromethyl)benzoyl group at C-2' is replaced by a hydrogen atom.49
.png?revision=1&size=bestfit&width=395&height=215)
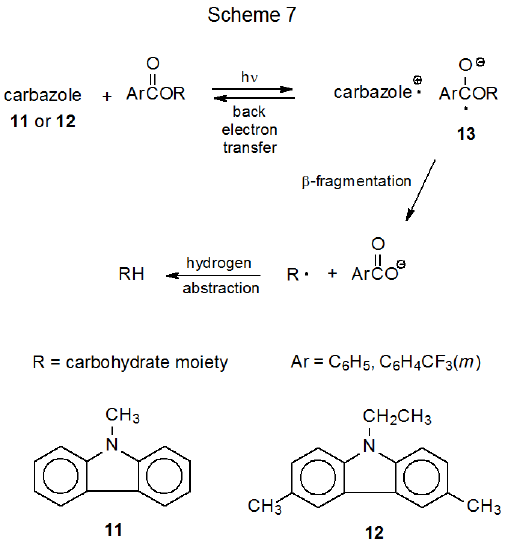
When reaction is conducted in the presence of Mg(ClO4)2, it is possible to replace even an unsubstituted benzoyloxy group with a hydrogen atom (eq 1450).50–52 Magnesium perchlorate affects this reaction by hindering back electron transfer, a process that competes with the fragmentation of the radical anion 13 (Scheme 7). Another factor that affects the reaction of a benzoyloxy group is the choice of the electron donor; thus, replacing N-methylcarbazole (11) with 3,6-dimethyl-9-ethylcarbazole (12) causes deoxygenation to take place more rapidly.50 Compound 12 is superior to 11 because it forms a more stable radical cation upon electron transfer.50,53,54
.png?revision=1&size=bestfit&width=425&height=203)