6.7: ¹H NMR Spectra and Interpretation (Part II)
- Page ID
- 359599
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)6.7.1 Integration of Signal Areas
The computer in the NMR instrument can be instructed to mathematically integrate the area under a signal or group of signals. The signal integration process is very useful in 1H NMR spectrum, because the area under a signal is proportional to the number of protons to which the signal corresponds.
The Fig. 6.7a is the 1H NMR spectrum of 1,4-dimethylbenzene with integration line (blue lines). The integration line generated by the computer is always in curve shape that resemble steps. The integration numbers are also generated by the computer together with the curve, that show the relative area of each signal (the integration numbers in the actual spectra are usually with decimals, whole numbers are shown here for simplicity).
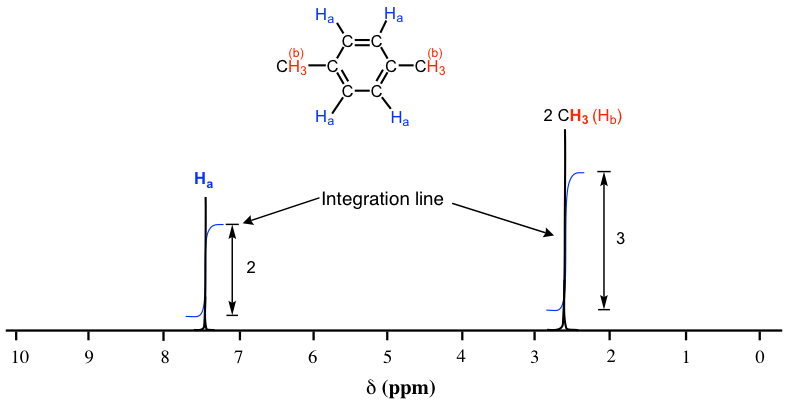
As we discussed earlier, the molecule of 1,4-dimethylbenzene has two sets of equivalent protons: the four aromatic (Ha) protons and the six methyl (Hb) protons. The integration of the area under the peak at 2.6 ppm is 1.5 times greater than the area under the peak at 7.4 ppm. Please note that the integration number show the relative ratio of the number of protons, not the actual number. The ratio 3 to 2 here matches the ratio of actual number 6 to 4. This integration information, along with the chemical shift knowledge we have learned before allow us to assign the peaks: peak at 7.4 ppm correspond to protons (Ha) on the benzene ring, and the peak at 2.6 ppm correspond to two methyl groups (Hb).
6.7.2 Signal Splitting (Coupling)
In the 1H NMR spectra that we have seen so far, each set of protons generates a single NMR signal. This is not that common for 1HNMR actually. In fact, the 1H NMR spectra of most organic molecules contain signals that are ‘split’ into two or more peaks that is called splitting (or coupling). The spectra with peak splitting may looked more complicated, however, this splitting behavior provides very useful information about the structure of a compound.
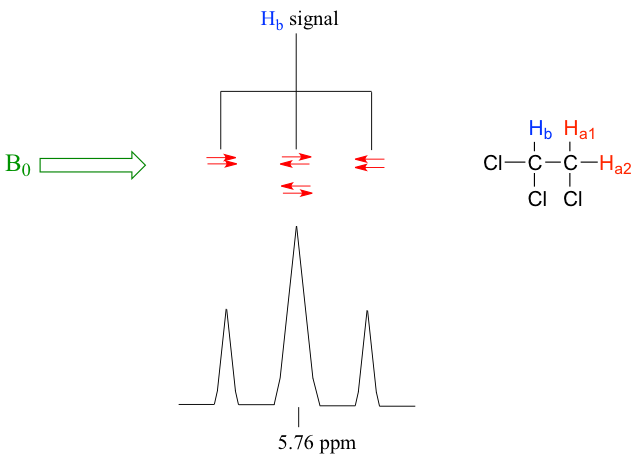
Let’s consider the spectrum for 1,1,2-trichloroethane (Fig. 6.7b). In this and in other spectra to follow, the expansions of individual signals are shown so that the signal splitting patterns are recognizable.
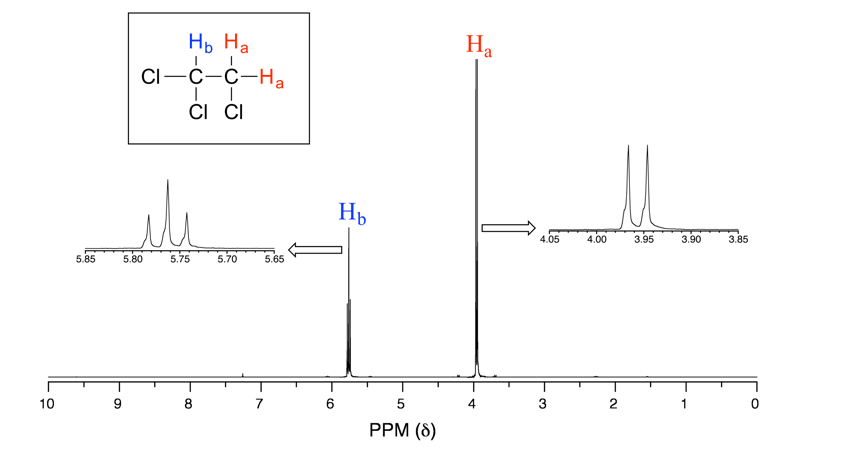
The signal at 3.96 ppm, corresponding to the two Ha protons, is split into two peaks of equal height (and area) – this is referred to as a doublet. The Hb signal at 5.76 ppm, on the other hand, is split into three peaks, with the middle peak higher than the two outside peaks and the integration ratio between the three peaks is 1:2:1, such splitting signal is called a triplet.
Signal splitting is caused by spin-spin coupling, a term that describes the magnetic interactions between non-equivalent hydrogen atoms that are with 2 or 3 bonds of the hydrogens producing the signal. The nearby protons have magnetic moment that can be either against or with the external magnetic field, therefore splits the energy levels of the protons whose signal is being observed, and result in the splitting of the signal into multiple peaks (the terms ‘splitting’ and ‘coupling’ are often used interchangeably when discussing NMR).
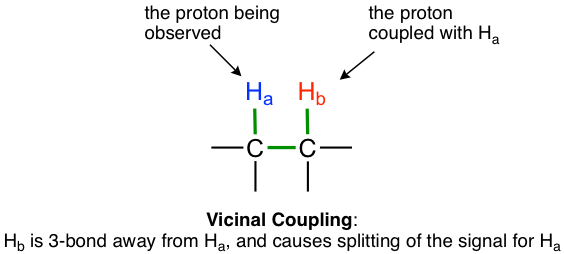
The most typical coupling we observed in this course is from non-equivalent vicinal hydrogens that are 3 bonds away, that is the hydrogens on adjacent carbons. This is also called vicinal coupling or three-bond coupling.
A simple rule that applies for predicting the number of peaks (or splitting pattern) expected from coupling and the rule in 1H NMRis:
number of peaks = n + 1
(n is the number of vicinal non-equivalent hydrogens)
We will exam the splitting pattern with different number of n:
- When n=0, the signal is a singlet, or has only one peak, as the signals observed in Fig. 6.6d and Fig. 6.7a.
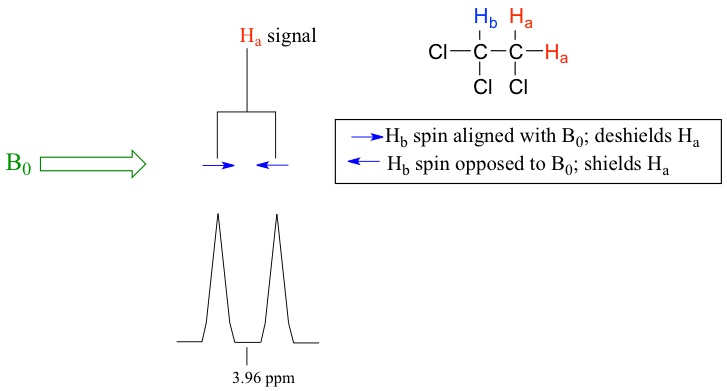
- When n=1, the signal is a doublet with two peaks. The area ratio of the two peaks for a doublet is 1:1. The space between the two peaks is called coupling constant, Jab, measured in Hz.
For the example of compound 1,1,2-trichloromethane, the signal of Ha protons fits into this situation. With only one vicinal proton, Hb, on the adjacent carbon, the signal of Ha show as a doublet.
- When n=2, the signal is a triplet with three peaks. The three peaks of triplet has the ratio of the area as 1:2:1.
In the same compound 1,1,2-trichloromethane, the signal of Hb proton fits into this situation. With two vicinal protons, 2Ha, on the adjacent carbon, the signal of Hb show as a triplet.
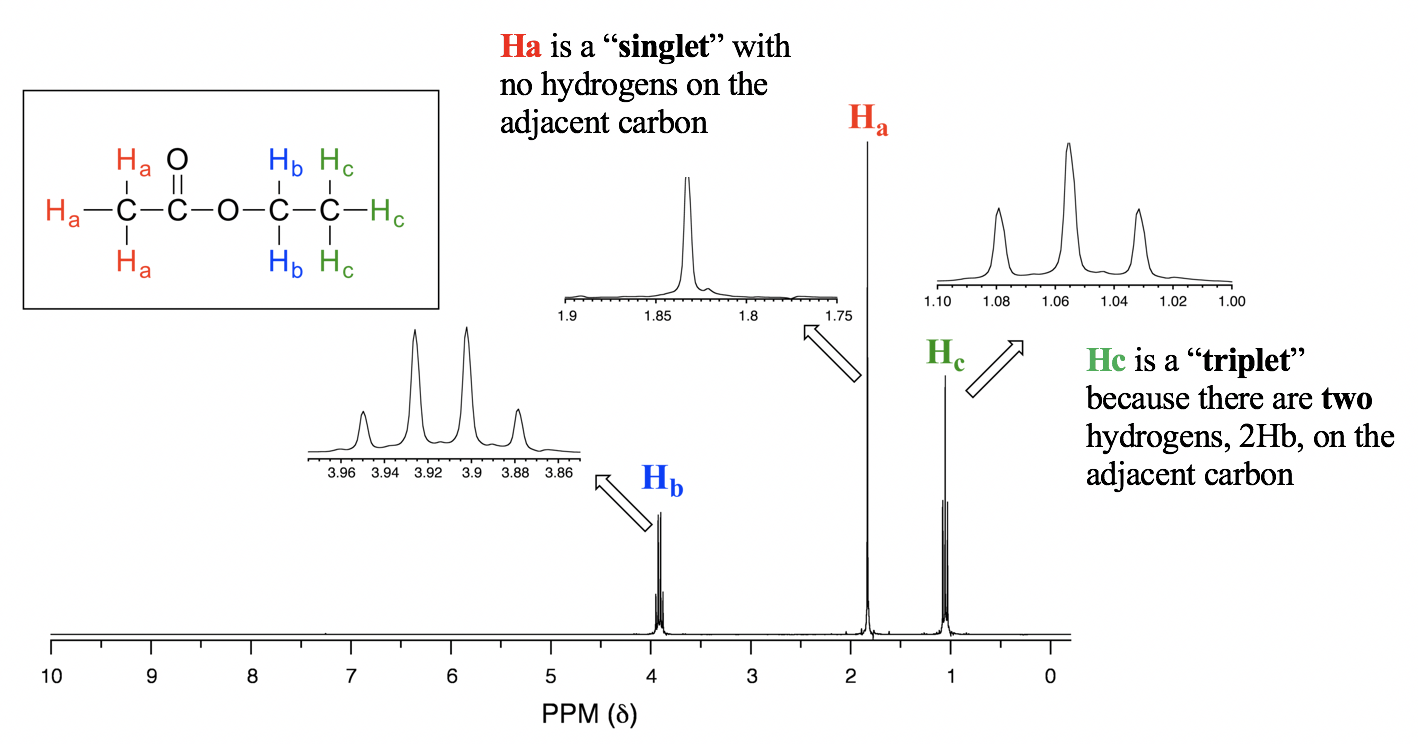
- When n=3, the signal is a quartet, that means four peaks. The four peaks of quartet has the area ratio of 1:3:3:1.
For the spectrum of ethyl acetate (Fig. 6.7e), the signal of Hb is a quartet, because there are three vicinal protons 3Hc on the adjacent carbon. Please note that the carbon with Hb connected with oxygen on the other side, and there are no hydrogen atoms on that oxygen atom, so only the coupling with three vicinal protons apply.
- When n≥4, the signal can be called a multiplet. Theoretically, with n increase the signal split into more peaks and the total number of peaks is “n+1”. However, the small peaks on the sides may or may not be able to be observed since they might be merged into noise. The signal with more than four peaks are generally called as a multiplet, and it is not that critical to tell exactly how many peaks involved in a multiplet.
Extra notes about signal splitting:
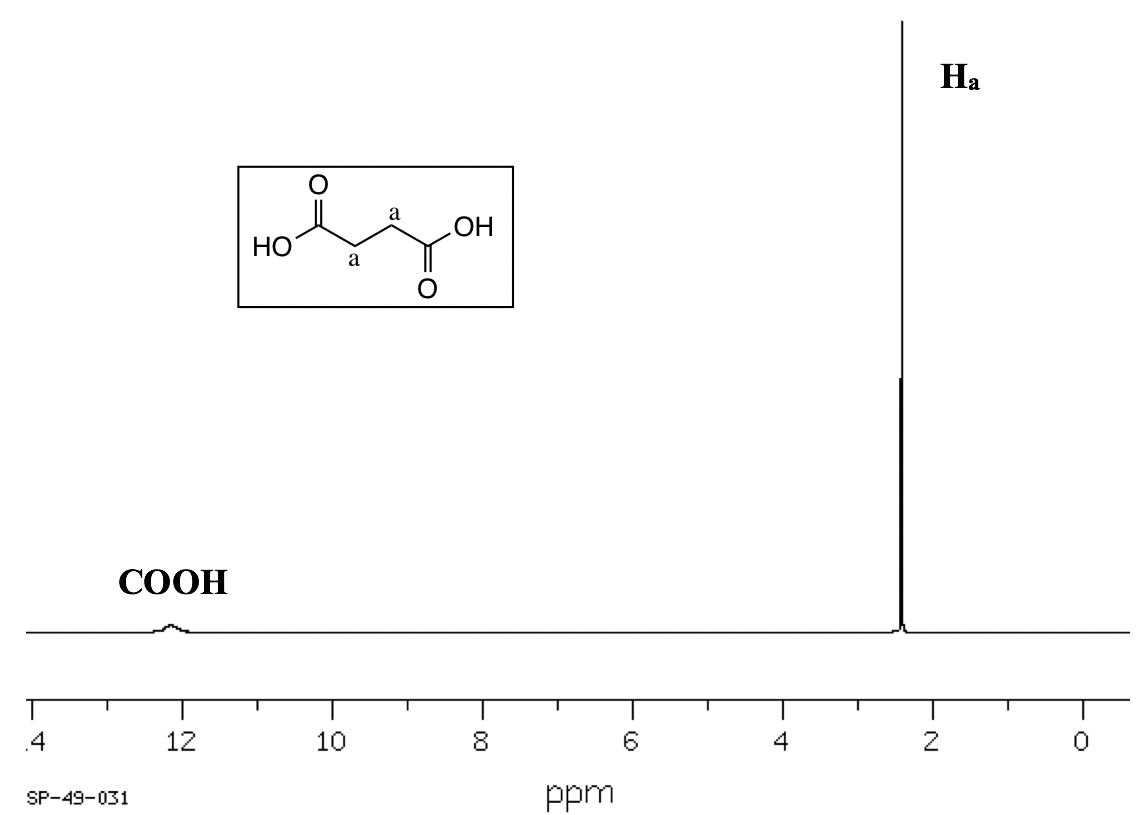
- Splitting (coupling) only occurs between nonequivalent protons. For equivalent protons, there is no coupling. In the spectrum of succinic acid (Fig. 6.7f) for example, the protons on the two middle carbons are equivalent (Ha), so there is no coupling between them and they show a singlet.
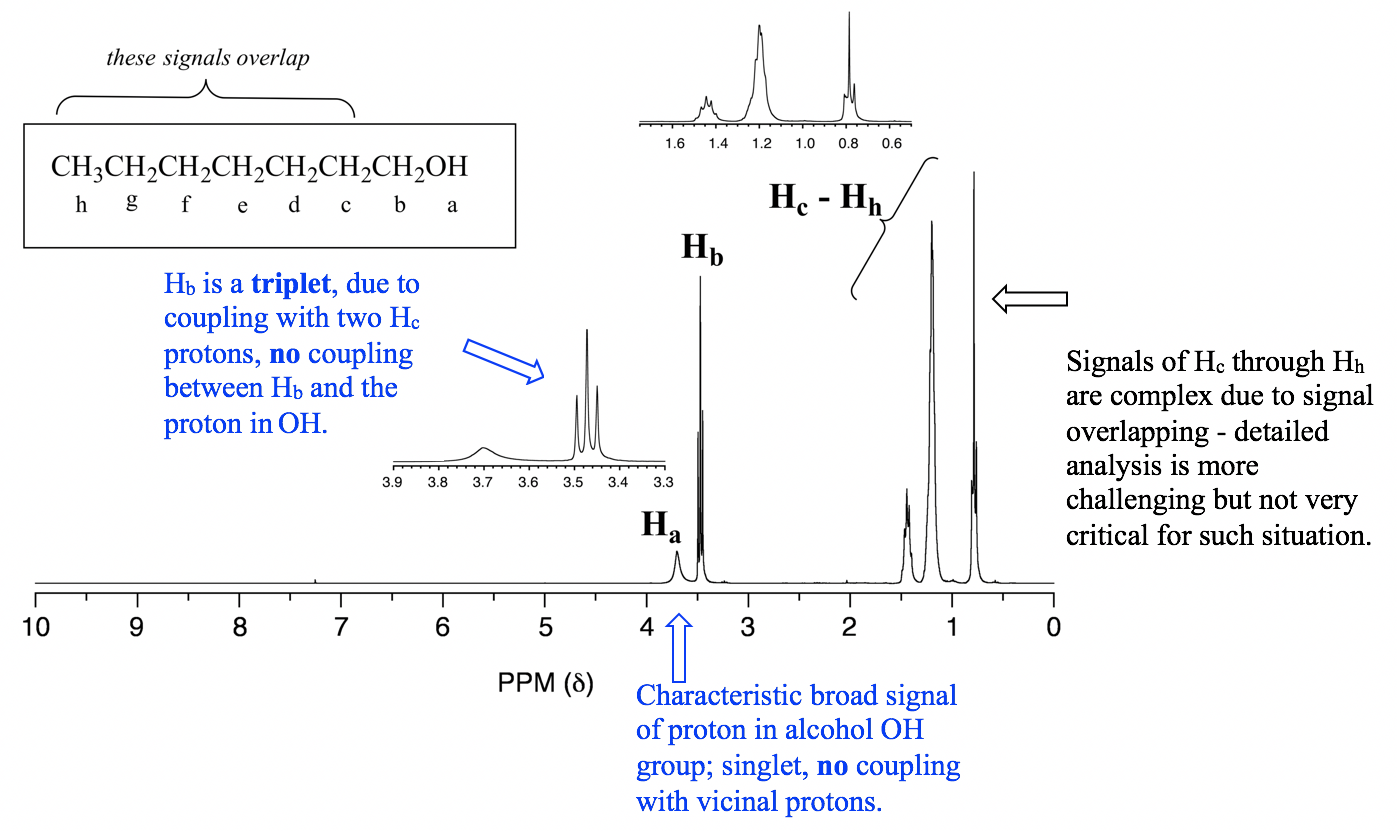
2. Protons in OH or NH generally do not couple with vicinal hydrogens. OH and NH protons are acidic enough to rapidly exchange between different molecules, so the neighboring protons never actually ‘feels’ their influence. See the specific example of 1-heptanol spectrum in Fig. 6.7g.
6.7.3 1H NMR Practice
Signal assignment based on the given structure
With the structure of a compound given, we can apply all the knowledge about 1H NMR to assign the signals in the spectrum, that is to identify a certain signal comes from which hydrogen(s).
Examples
Match the 1H NMR spectrum below to its corresponding compound, and assign all of the signals.
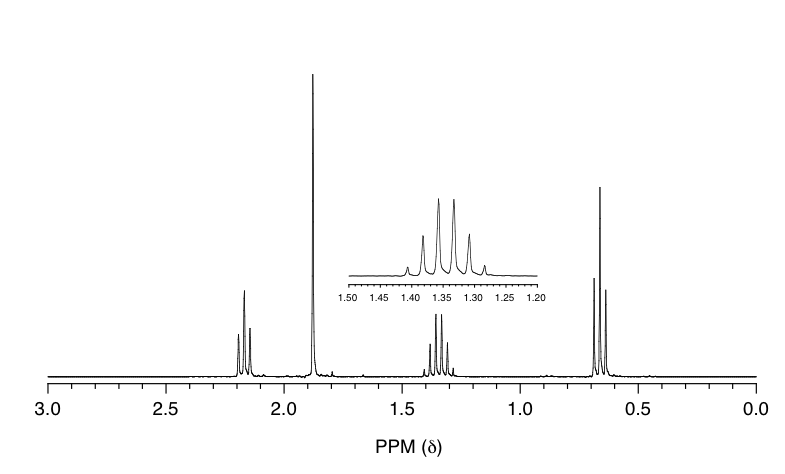
a) cyclopentanone b) 3-pentanone c) butaldehyde d) 2-pentanone
e) 4-heptanone f) 1-butene
Approach: It is good idea to draw the structure of each compound and try to see which matches to the spectrum.
The spectrum has four signals: triplet (~0.7 ppm), multiplet (~1.4 ppm), singlet ( ~1.9 ppm) and triplet (~2.2 ppm). Based on the structure of each compound, compound c), d) and f) should have four signals in the 1H NMR spectrum.
- There is no signals at about 9 ppm for the aldehyde hydrogens in the spectra, so the spectrum is not for compound c) , butaldehyde.
- There is no signals at about 4~5 ppm for the alkene hydrogens in the spectra, so the spectrum is not for compound f) , 1-butene.
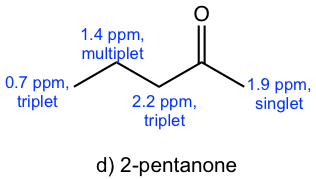
- The signals in the spectrum match with what are expected for compound d), 2-pentanone.
Solution: The spectrum is for 2-pentanone.
Structure Determination based on 1H NMR spectrum
For an advanced level of practice, we are supposed to be able to determine the exact structure of a compound with 1H NMR spectrum given (and other necessary information). As we have learned, there are a lot valuable information about the structure of a compound can be obtained from an 1H NMR spectrum. For a summary, analyzing the four features of the spectrum is critical to elucidate the structure of a compound:
- The number of signals indicate how many different sets of protons there are in the molecule;
- The chemical shift of the signal tells us about the electronic environment of each set of protons;
- The integration under each signal provides information about how many protons there are in the set being measured (keep in mind that the integration values are for the ratio, not actual number of protons);
- The splitting pattern of each signal tells about the number of protons on atoms adjacentto the one whose signal is being measured.


