10.5: Transition States
- Page ID
- 215753
One of the simplest types of organic reactions is the Sn2 reaction (Ch. 6 in the Wade textbook). In this reaction a bond is being broken as another bond is formed almost simultaneously. Atom A moves towards molecule B-C. As A bonds to B, C is breaking away.
\(A + B-C \rightarrow A-B + C\)
If we were to view this process “frame by frame,” we would see an energy diagram showing each stage of the process and the corresponding energy levels associated with each stage of the process. This is one of the simplest types of reaction profiles.
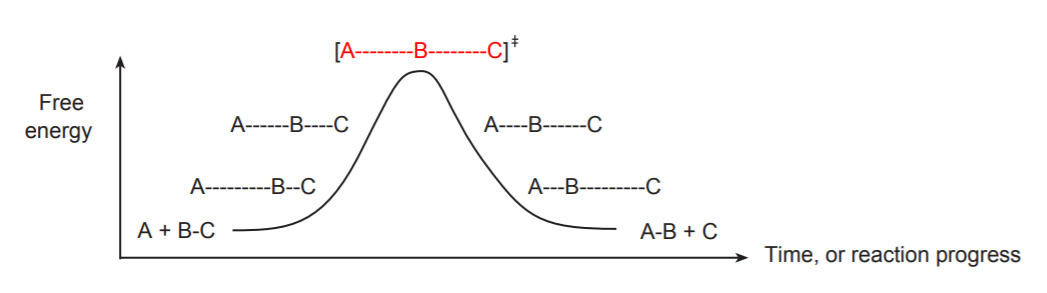
A point of particular interest in this reaction profile is the energy maximum. The structural species that exists at this point is called a transition state (shown in red). It is customary to enclose this structure in brackets, with a double dagger at the top right. The transition state represents a point of such high energy that it cannot possibly exist for any length of time. As an analogy, think of a rubber band that gets stretched to the point where it’s about to snap. The state of maximum energy occurs a fraction of a millisecond before it snaps. The rubber band cannot stay in that state for any length of time. Either it relaxes and goes back to its original state, or it snaps. Either way it must go down to a more stable state. Therefore, transition states cannot be physically or experimentally observed, much less studied. Their existence and their structure must be inferred from other information, especially that which pertains the surrounding states.


