10.6: Covalent Bond Cleavage- Outcomes and Reaction Intermediates
- Page ID
- 215754
What we refer to as the reactants and the products of a chemical reaction represent stable substances that can exist for a finite period of time under standard conditions. They can typically be kept in containers without significant decomposition as long as appropriate measures are observed. In other words, the beginning and the end of a chemical reaction (reactants and products), represent the most stable states that can exist in reference to the reaction profile. Any other intermediate structures or states must be of higher energy, or lower stability. Two types of points in particular merit special attention: energy maxima and energy minima. We’ve already discussed energy maxima above in relation to transition states.
An energy minimum that occurs between reactants and products represents a reaction intermediate. Reaction intermediates represent structures that do have some degree of stability, but not as much as reactants or products. They can live long enough that they can in principle be detected, observed, and even studied under the right conditions. However they are not stable enough to keep for indefinite periods of time.
In organic chemistry we are mainly concerned with three types of reaction intermediates that result from breaking a covalent bond in two different ways, referred to as homolytic cleavage and heterolytic cleavage. In the former, the electrons that make up the covalent bond get equally distributed between the atoms that make up the bond. In heterolytic cleavage, one of the two atoms gets the two electrons and usually develops a negative charge. The other atom gets no electrons and typically develops a positive charge. Homolytic cleavage gives rise to the formation of free radicals, that is, neutral species that carry an unpaired electron. Heterolytic cleavage gives rise to the formation of ions, that is, species that carry a positive or a negative charge.
In the curved arrow formalism, the movement of single electrons is indicated by half-headed arrows. The movement of electron pairs is indicated by full-headed arrows. The following examples illustrate the outcomes of homolytic and heterolytic bond cleavage.
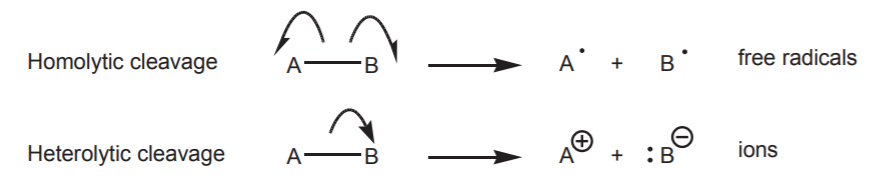
When the atom in question is a positively charged carbon, the resulting species is called a carbocation. If it is negatively charged, it is called a carbanion. The following represent all three species with carbon as the central atom.
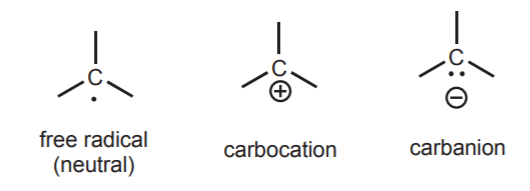
These three species are the most common types of reaction intermediates that occur in organic reactions. Again, they occur at energy minima located between reactants and products in the reaction profile. The hybridization state of the central carbon in each of these intermediates is important. This and other structural features are further discussed in your organic chemistry textbook (Section 4-16 in the Wade textbook).


