10.4: Stability of the Allyl Radical - Resonance Revisited
- Page ID
- 31497
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- explain the stability of the allyl radical in terms of resonance.
- explain the difference between resonance and tautomerism.
- write an equation for the reaction of an unsymmetrical alkene with N-bromosuccinimide.
- draw the structure of each of the possible products that could be obtained from the reaction of a given unsymmetrical alkene with N-bromosuccinimide, and predict which product will predominate.
- explain the formation of more than one product from the reaction of N-bromosuccinimide with a given unsymmetrical alkene.
- explain the observed product ratio when a given unsymmetrical alkene is treated with N-bromosuccinimide.
Make certain that you can define, and use in context, the key terms below.
- delocalized
- resonance forms
- resonance hybrid
You will have encountered the concept of resonance if you have taken general first-year chemistry course. You should also briefly review Section 2.5.
When we can represent a species by two or more different Lewis or Kekulé structures, neither of which represents the true structure of the species, these structures are referred to as resonance forms. A common example used in general chemistry courses to illustrate the concept of resonance is ozone, O3. The two resonance forms of ozone may be represented as follows:
The concept of resonance is quite important, and will be used frequently throughout the remainder of this course. The guidelines below may assist you in drawing resonance contributors.
- Resonance occurs whenever a molecule, radical or ion can be represented by two or more structures differing only in the arrangement of electrons (no atoms may be moved).
- The true structure of a species is a hybrid of the resonance contributors and is more stable (i.e., lower in energy) than any of the contributors.
- The most important contributors are those containing the most covalent bonds. Another way of saying the same thing is that the most important contributors have the least amount of charge separation.
- Contributors in which all the atoms (except hydrogen) have a complete octet (i.e., are surrounded by eight electrons) are particularly important.
In the previous section we discussed the allylic bromination of a symmetrical alkene with NBS such as this cyclopentene, which affords one product.
However, with an unsymmetrical alkene and the delocalized unpaired electron forming various allylic resonances, several products are possible. For example, the NBS bromination of 4-methyl-cyclohexene leads to three products.
The geometry and relative stability of carbon radicals
As organic chemists, we are particularly interested in radical intermediates in which the unpaired electron resides on a carbon atom. Experimental evidence indicates that the three bonds in a carbon radical have trigonal planar geometry, and therefore the carbon is considered to be sp2-hybridized with the unpaired electron occupying the perpendicular, unhybridized 2pzorbital. Contrast this picture with carbocation and carbanion intermediates, which are both also trigonal planar but whose 2pz orbitals contain zero or two electrons, respectively.
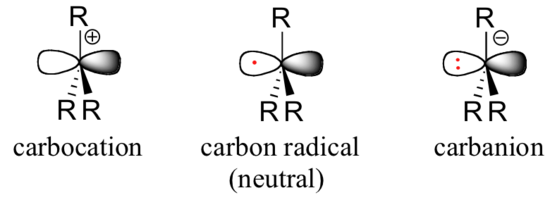
The trend in the stability of carbon radicals parallels that of carbocations (section 8.4B): tertiary radicals, for example, are more stable than secondary radicals, followed by primary and methyl radicals. This should make intuitive sense, because radicals, like carbocations, can be considered to be electron deficient, and thus are stabilized by the electron-donating effects of nearby alkyl groups.
Benzylic and allylic radicals are more stable than alkyl radicals due to resonance effects - an unpaired electron can be delocalized over a system of conjugated pi bonds. An allylic radical, for example, can be pictured as a system of three parallel 2pz orbitals sharing three electrons. With two resonance forms, the allylic radical is electronically symmetrical. Due to resonance hybrid theory, neither structure is correct, but instead the structure lies somewhere between the two resonance forms. Another way to phrase this is that the unpaired electron is delocalized across all the carbon atoms through the pi system and not localized on one site. The more resonance structures, the more stable the molecule. This is why the allylic radical is more stable than the alkyl radical.
Because the allylic radical is symmetrical, a reaction can occur on either side. Therefore if reacting with bromine, the bromination could occur on either end of the allylic radical. When the allyl radical is symmetrical, this yields the same product. However, if you have an unsymmetrical allyl radical, it would lead to a mixture of products and not necessarily in equal amounts. This is because the intermediate radical is unsymmetrical. The reaction will occur at the less hindered site. An example would be 1-octene as a starting material in a bromination. The products the reaction would yield would be 3-bromo-1-octene and 1-bromo-2-octene.
Further Reactions
The products from allylic bromination reactions can easily be converted into dienes by elimination using a base. If the alkyl chain is long enough, alkenes can be converted to dienes through a two-step process: allylic bromination followed by elimination.
1) The following reaction shows the major product. Explain why this would be the final product and why the 2° bromo product is not the major product.
2) Predict the products of the following reactions:
- Answer
-
1) The product (A) is a 1° halogen which is more predominant product even though the (B) had a better transition state with a 2° radical. The 1° radical intermediate is not as sterically hindered.
2)

