22.3: Alpha Halogenation of Aldehydes and Ketones
- Page ID
- 36414
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate the alpha halogenation of aldehydes and ketones.
- identify the product formed from the alpha halogenation of a given aldehyde or ketone.
- identify the carbonyl compound, the reagents, or both, needed to prepare a given α‑halogenated aldehyde or ketone.
- illustrate the importance of the alpha halogenation of carbonyl compounds as an intermediate step in the synthesis of α,β‑unsaturated aldehydes and ketones.
- write a detailed mechanism for the acid‑catalyzed halogenation of a ketone.
- describe the evidence provided by kinetic experiments supporting the suggestion that the acid‑catalyzed, alpha halogenation of ketones proceeds via the rate‑determining formation of an enol.
Note: α‑bromo ketones are a good starting material to generate α,β‑unsaturated ketones by dehydrobromination.
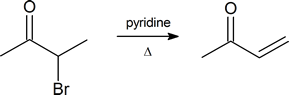
Aldehydes and ketones can substitute an α-hydrogen for a halogen atom in the presence of an acid. This reaction takes place using acid catalyzed tautomerization to form a nucleophilic enol, which then reacts with an electrophilic halogen (Cl2, Br2 or I2). Because an enol intermediate is used, a racemic mixture of products can be produced. A particularly useful variation of this reaction uses bromine in an acetic acid solvent. The α-bromo substituted product can then be readily transformed into an α, β‑unsaturated carbonyl through reaction with pyridine and heat.
General Reaction (α substitution)
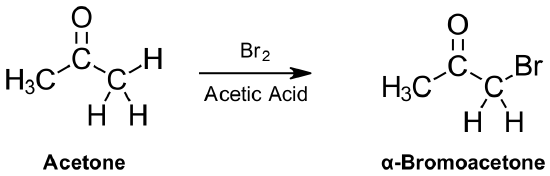
Acid Catalyzed Mechanism
The mechanism begins with protonation of the carbonyl oxygen followed by removal of an α-hydrogen to form the enol. Lone pair electrons from the enol oxygen move to form a carbonyl while the pi electrons from the double bond attack the halogen forming an oxonium ion intermediate with a C-X sigma bond in the α-position. Deprotonation of the oxonium ion intermediate provides the α-halogen substituted product and regenerates the acid catalyst.
1) Protonation by the acid catalyst
2) Removal of an α-hydrogen to form the enol. This step is slow and represent the rate determine step.
3) Nucleophilic attack on the halogen
4) Deprotonation
Experimental Evidence of the Enol Intermediate
This reaction was the focus of one of the first mechanistic investigations in organic chemistry. In the early 1900's chemist Arthur Lapworth showed that the rates of chlorination, bromination, and iodination of acetone were all the same. Also, it was shown that the rates for all three halogenation reactions were first-order with respect to acetone and the acid catalyst but independent of the halogen concentration (overall second-order for the mechanism). The rate law expression for the α-halogenation of a ketone can be given by:
rate = k [ketone] [H+]
This implies that the halogen participates in the mechanism through a fast step which occurs after the rate-determining step. These observations led Lapworth to theorize that the rate-determining step of the mechanism involves converting acetone to a more reactive form. The fact that the substitution occurs on the α-carbon led Lapworth to propose that the more reactive form was an enol tautomer of acetone.
Synthetic Uses for α-Halogenated Carbonyls
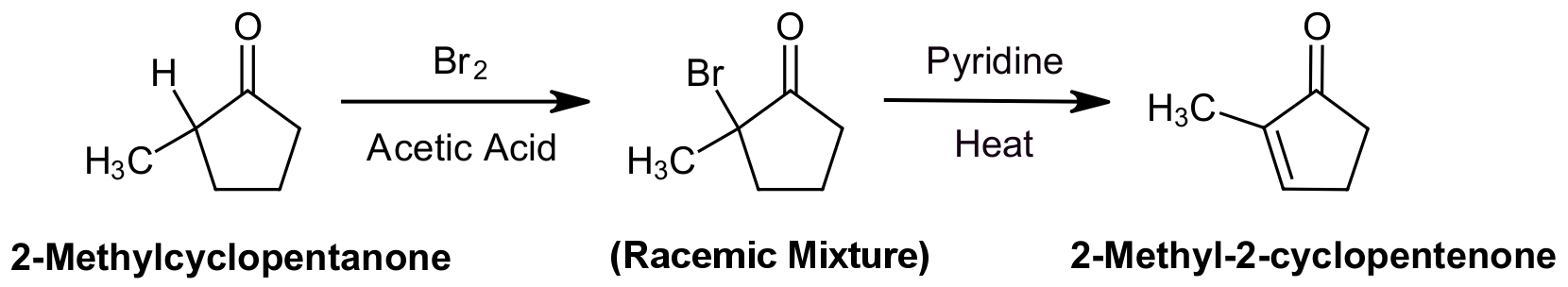
The product of an α-bromination can be converted to an α, β‑unsaturated carbonyl by reaction with pyridine and heat which causes the elimination of H and Br. This reaction takes place by an E2 elimination mechanism and creates a C=C double bond which is conjugated with the carbonyl. In order to promote an E2 reaction, a sterically hindered base, pyridine, is often used.
An example of this reaction involves the α-bromination of 2-methylcyclopentanone to form 2-bromo-2-methylcyclopentanone. Because enol tautomers prefer to form on the more substituted α-carbon, α-bromination also occurs on the more substituted α-carbon. Although the enol intermediate causes a racemic mixture of the α-brominated compound to form, it is irrelevant because the chiral carbon is subsequently converted to an achiral alkene. Subsequent reaction with pyridine and heat forms the α,β‑unsaturated ketone, 2-methyl-2-cyclopentenone.
Deuterium Exchange
More evidence for the formation of an enol intermediate was provided using a reaction called deuterium exchange. Deuterium is an isotope of hydrogen which contains one proton and one neutron. Due to the acidic nature of α-hydrogens, they can be exchanged with deuterium by reaction with the isotopic form of water, D2O (deuterium oxide-heavy water). The process is accelerated by addition of the deuterium equivalent of a strong acid, such as deuterium chloride (DCl), which quickly reacts with D2O to form D3O+, the deuterium equivalent of hydronium (H3O+). If an excess of D2O is used, the exchange process continues to the end result of all α-hydrogens present in a given compound being replaced with deuterium. Deuterium exchange is an effective method for introducing an isotopic label into a molecule. Also, deuterium does not appear in 1H NMR, so deuterium exchange can help determine peak assignments.
General Reaction (Deuterium exchange)
Mechanism in Acidic Conditions
The mechanism for deuterium exchange is virtually the same as keto-enol tautomerism under acidic conditions, as shown in Section 22.2. The only difference is that when the keto tautomer is reformed a deuterium is placed in the α-position.
1) Formation of an enol
2) α-Deuteration
3) De-deuteration to form the keto tautomer
A simple method for determining the number of α-hydrogen in a compound is through reaction of D3O+. The reaction product is then isolated and its molecular weight is determined by mass spectrometry. For example, if cyclopentanone is reacted with D3O+, the isolated product has a molecular weight of 88 g/mol. Please explain how this method works and how many α-hydrogens cyclopentanone is predicted to have.
Solution
During acid catalyzed deuterium exchange each α-hydrogen in the compound is replaced with a deuterium. For each proton (AW = 1) replaced with a deuterium (AW = 2) the molecular weight of the compound is increased by one. Since cyclopentanone has a molecular weight of 84 g/mol and the isolated product has a molecular weight of 88 g/mol it can be predicted that cyclopentanone has four α-hydrogens.
Kinetic investigations into the mechanism of this reaction provided further evidence for the formation of a reactive enol intermediate. It was shown that the rate of deuterium exchange was the same as the rate of halogenation for ketones. This implies that both reactions have a common intermediate involved in the rate determining step of their mechanism, an enol.
Exercises
1) Please draw the products of the following reactions
2)Draw out the mechanism for the following reaction.
3) How might you form 2-hepten-4-one from 4-heptanone?
4) Show the products of the following reactions:
5) The following compound was reacted with D3O+. The only signals that could be found in the 1H NMR spectrum of the product were at 3.9 ppm (3H) and 6.6-6.9 ppm (4H). Please explain the results of the NMR.
Solutions
1)
2)
3) [1) Br2, H3O+; 2) Pyridine, Heat]
4)
5) A deuterium exchange reaction occurred. All of the alpha-hydrogens in the molecule have been exchanged with deuterium. Because detueriums do not appear in a typical 1H NMR, only the remaining hydrogens appear.

