18.9: Spectroscopy of Ethers
- Page ID
- 36373
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- use the 1H NMR spectrum of an unknown ether or epoxide to determine its identity.
- identify the approximate chemical shift expected for protons attached to the carbon atoms that are bonded to oxygen in an ether or an epoxide.
Infrared Spectroscopy
Ethers and epoxides typically have a strong C-O stretch between 1000 and 1300 1/cm. Because this absorption appears in the fingerprint region of the IR is can be difficult to assign. In addition to the C-O peak, it is helpful to note if an IR spectrum has no C=O or O-H stretch peaks to confirms it is not aldehyde, ketone, or alcohol.
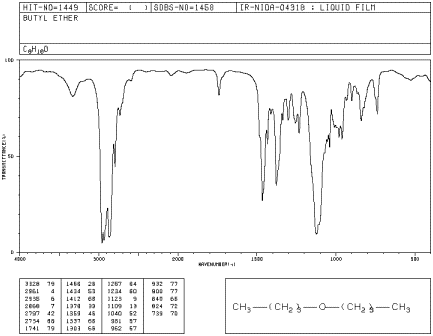
If you look at an IR spectrum of dibutyl ether, you will see:
- there are the usual sp3 C-H stretching and CH2 bending modes at 2900 and 1500 cm-1.
- there is a strong peak near 1100 cm-1. This peak is due to the C-O stretching vibration.
Figure IR7. IR spectrum of dibutyl ether. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
Although thios and sulfides do have a weak C-S stretch between 710 and 570 1/cm, the absorption is very difficult to assign. In addition thios have a weak S-H stretch at 2600-2550 1/cm.
1H NMR Spectroscopy
- Hydrogens on carbon adjacent to the ether show up in the region of 3.4-4.5 ppm.
- Similar peaks in epoxides are shifted to a slightly higher field than other ethers. Hydrogens on carbons in and epoxide appear in the region of 2.5 to 3.5 ppm.
- Hydrogens on carbons adjacent to the sulfur in sulfides and thiols appear in the region of 2.0 to 2.5 ppm.
- The SH hydrogen of a thiol typically appears in the region of 1.3-1.5 ppm.
The 1H NMR spectrum of dipropyl ether shows three signals with the triplet at 3.37 ppm assigned to the -CH2- beside the ether and the other two signals upfield (1.59 and 0.93 ppm). Notice the protons closer to the electron withdrawing oxygen atom are further downfield indicating some deshielding. Protons at (A) and (C) are each coupled to two equivalent (B) protons. So, each of these signals appears as a triplet. The (B) protons in turn are coupled to a set of two and three equivalent protons and appears as a sextet. Source: SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, 28 June 2017).
Dipropyl ether
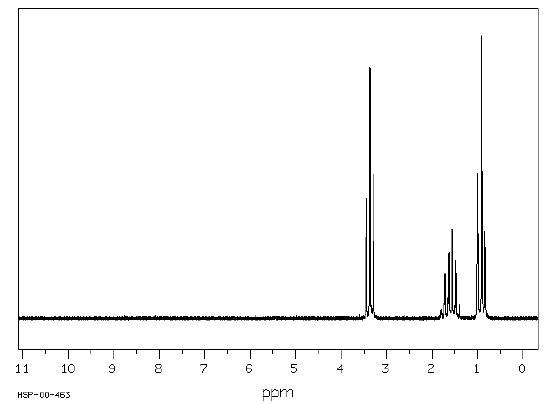
Source: SDBSWeb : http://sdbs.db.aist.go.jp
2-Methyloxirane
Source: SDBSWeb : http://sdbs.db.aist.go.jp
The methylene protons of this epoxide are diastereotopic and appear as two separate peak. When looking at the 3D structure of 2-methyloxirane it is clear that each methylene hydrogen is distinctly different. Also, hydrogens attached to the carbons in eposxides tend to display complex splitting patterns (Sections 13-7 and 13-8).
A 3D Interactive Model of 2-Methyloxirane
Ethanethiol
Source: SDBSWeb : http://sdbs.db.aist.go.jp
Note that in this 1H NMR spectra the SH proton is actively involved in splitting. The SH proton peak at 1.39 ppm is split into a triplet and the methylene peak at 2.55 ppm is split into a quintet.
13C NMR Spectra
- Carbons adjacent to the ether appear in the region of 50-80 ppm.
- Carbons that are part of the epoxide appear in the region of 40-60 ppm.
- Carbons adjacent to the sulfur in sulfides and thiols appear in the region of 20-40 ppm.
Dipropyl ether
Source: SDBSWeb : http://sdbs.db.aist.go.jp
2-Methyloxirane
Source: SDBSWeb : http://sdbs.db.aist.go.jp
Ethanethiol
Source: SDBSWeb : http://sdbs.db.aist.go.jp
Mass Spectra
Ethers, sulfides, and epoxides all have similar fragmentation patterns in mass spectra with a few subtle variations.
Ethers
- The M+ is typically weak or absent
- They primarily undergo alpha-cleavage to produce [H2C=O-R]+
- They can also undergo an inductive cleavage to produce R+
Epoxides
- The M+ is typically weak or absent
- They primarily undergo alpha-cleavage to produce an alkyl radical
Sulfides
- The M+ is typically stronger than the corresponding ether
- They primarily undergo alpha-cleavage to produce an alkyl radical
- They can also undergo an inductive cleavage to produce R+
Fragmentation Patterns
alpha-cleavage
inductive cleavage