16.9: Oxidation of Aromatic Compounds
- Page ID
- 31581
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to describe the oxidation of an alkylbenzene to a carboxylic acid.
- identify the reagents required to oxidize a given alkylbenzene to a carboxylic acid.
- identify the product formed from the side-chain oxidation of a given alkylbenzene.
- identify the aromatic compound needed to produce a given carboxylic acid through side-chain oxidation.
- write the equation for the bromination of an alkylbenzene side chain.
- identify the reagents and conditions necessary to bring about bromination in the side chain of an alkylbenzene.
- identify the product formed when a given alkylbenzene undergoes side-chain bromination.
- identify the alkylbenzene needed to prepare a given benzylic bromide by radical substitution.
- write the mechanism for the radical substitution at the benzylic position of an alkylbenzene.
- explain the stability of benzylic radicals in terms of resonance, and draw the resonance contributors of a given benzyl radical.
- explain, and illustrate with appropriate examples, the importance of benzylic bromides as intermediates in organic syntheses.
- arrange a given series of radicals (including benzylic type radicals) in order of increasing or decreasing stability. (Review Section 10.3 if necessary.)
Make certain that you can define, and use in context, the key terms below.
- benzylic oxidation
- benzylic position
- side-chain oxidation
As you can see from the examples, no matter what the length of the alkyl group in the arene substrate, the product is always a one-carbon carboxyl group. Thus, the benzylic carbon atom has been oxidized and the term benzylic oxidation is appropriate. The term side-chain oxidation is also commonly used.
In alkylbenzenes, the carbon atom which is attached to the aromatic ring is particularly reactive. Reactions taking place at this carbon atom are said to occur at the benzylic position.
You may wish to review Section 10.3 to remind yourself about allylic bromination using N-bromosuccinimide.
Benzylic halides undergo the typical reactions of alkyl halides; thus, you can expect to see such compounds used frequently in multistep syntheses.
Note that we have adopted the terminology given below.
Any compound of the type

(where X = halogen) will be referred to as a “benzylic halide.”
Compounds of the type
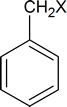
are actually called benzyl chloride, benzyl bromide, etc.
The compound

is called benzyl alcohol.
Oxidation of Alkyl Side-Chains
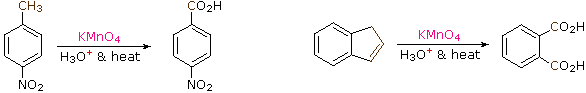
The benzylic hydrogens of alkyl substituents on a benzene ring are activated toward free radical attack, as noted earlier. Furthermore, SN1 SN2 and E1 reactions of benzylic halides, show enhanced reactivity, due to the adjacent aromatic ring. The possibility that these observations reflect a general benzylic activation is supported by the susceptibility of alkyl side-chains to oxidative degradation, as shown in the following examples (the oxidized side chain is colored). Such oxidations are normally effected by hot acidic permanganate solutions, but for large scale industrial operations catalyzed air-oxidations are preferred. Interestingly, if the benzylic position is completely substituted this oxidative degradation does not occur.
\[\ce{C6H5–\color{red}{CH2CH2CH2CH3} + KMnO4 + H3O^{+} + heat -> C6H5–\color{red}{CO2H} + CO2} \nonumber \]
\[\ce{p-(CH3)3C–C6H4–CH3 + KMnO4 + H3O6{+} + heat -> p-(CH3)3C–C6H4–CO2H} \nonumber \]
These equations are not balanced. The permanganate oxidant is reduced, usually to Mn(IV) or Mn(II). Two other examples of this reaction are given below, and illustrate its usefulness in preparing substituted benzoic acids.
Bromination of the Benzylic Carbon
The benzylic C-H bonds weaker than most sp3 hybridized C-H. This is because the radical formed from homolysis is resonance stabilized.
Resonance stabilization of the benzylic radical
Because of the weak C-H bonds, benzylic hydrogens can form benzylic halides under radical conditions.
NBS as a Bromine Source
NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the allylic bromination reaction.
.jpg?revision=1&size=bestfit&width=307&height=104)
Allylic Bromination Mechanism
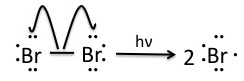
Step 1: Initiation
Once the pre-initiation step involving NBS produces small quantities of Br2, the bromine molecules are homolytically cleaved by light to produce bromine radicals.
Step 2 and 3: Propagation
Step 4: Termination
Predict the products of the following two reactions.
- Answer
-
The second one leads to no reaction because it requires a hydrogen just off the phenyl ring.
Consider a benzyl radical. Would it be more stable than an alkyl radical? Explain.
- Answer
-
Yes it would be more stable than an alkyl radical. The benzyl radical is stabilized through several resonance structures where the radical is moved through the ring via the pi system there.
How would you make the following molecule?
- Answer
-
The following is just one possibility.

