7.4: Synthesis
- Page ID
- 354668
Up to now, we have focused mostly on the reactivity of organic molecules, particularly from the standpoint of being able to predict reactivity from the structure of a particular compound. We have also discussed how spectroscopic techniques allow us to determine molecular structure. However, we have not really discussed another of the major areas of organic chemistry, that is: the design and synthesis of molecules, particularly those that may be expected to have biological activity (drugs of various sorts). Now that we have a fairly large repertoire of reactions to choose from, let us take a closer look at the kinds of decision-making that goes into designing molecular structures.
Typically, molecular synthesis involves a target structure, which may be an actual molecule, or it could be a substance that has particular properties: for example, the active site of an enzyme or a regulatory domain of a protein. To achieve specificity, the molecule to be synthesized must fit into the surface of the protein and influence its structure or catalytic activity. The better the fit, the more specific (higher affinity, fewer non-target interactions) interactions the drug will make. We will begin by thinking about how to go about the synthesis of a given molecule. Molecular synthesis is both an art and a science: it requires that you have at your fingertips a good collection of reactions you have organized in such a way as to make them accessible to you, but it also requires creativity and imagination. There is always more than one way to design a synthesis and, in reality, there are many setbacks and path changes since reactions may not go as planned, so alternative routes have to be considered. In this section, we will look at strategies that you might employ to design a synthesis of a target molecule.
Retrosynthetic analysis
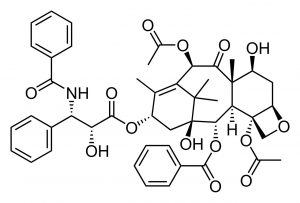
Retrosynthetic analysis is exactly what it sounds like: you begin with the target and move backwards one step at a time to identify what reactants and reagents could have produced the products. This is often the situation when you are dealing with a natural product that was originally isolated based on its biological activity. A classic example is the molecule Paclitaxel, which was isolated from the Pacific yew tree, Taxus bervifolia, based on its anti-cancer properties.[14]
The process of molecular synthesis can be broken down into a number of steps, but as we will see, depending on the nature of the task, we do not always approach synthesis in a linear manner.
Step 1: Identify the number of carbons in the target molecule and determine whether you will need (at some point in the synthesis) to make new carbon-carbon bonds.
Step 2: Identify what functional groups are present in the molecule. Functional groups are where the reactivity of any molecule lies and they give you a place to begin because you now know ways to produce that functional group.
Step 3: Identify which bonds are going to be made during the reaction in which the product is produced.
Step 4: This will allow you to work backwards to identify what the precursor is and (using your knowledge of functional group transformations) decide what reaction will product the product.
Step 5: Repeat until you reach a recognizable target material.
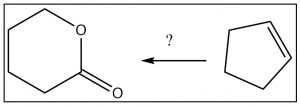
Let us approach the process with a (significantly simpler) target.[15] Let us design a synthesis of the lactone (cyclic ester) from cyclopentene. We begin by noting that both the starting material and the product have five carbons, which means we do not need to consider any carbon-carbon bond formation reactions. However, the route from cyclopentene to the lactone is not obvious, so let us go back one step at a time as outlined below.

So, we have the immediate precursor to the lactone, but how do we get that from cyclopentene? While you could continue moving backwards through the steps, the knowledge that you don’t have to construct the carbon skeleton makes a difference in the way that you might approach the problem. For example, if you look at the starting material, it is clear that there is no five-membered ring in the product and no alkene. Not only that, but at each end of the carbon chain is at least one oxygen. Is there a reaction that will allow us to open up that ring and, at the same time, introduce oxygens at each carbon? Yes! Recall that alkenes can be cleaved by ozonolysis (under oxidizing conditions), to give the dicarboxylic acid. If one of those carboxylate groups can be reduced to the alcohol, something that might well be be difficult to do in practice, the ring would cyclize spontaneously to give the lactone as shown below.
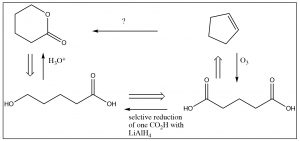
Note that, in this synthesis, we mixed up both retro and forward synthetic analysis to produce an overall synthetic pathway. This is where some of the art and imagination come into play. When you are trying to design a synthesis, it is a good idea to start from the product and work backwards—but at some point you may have to also work forward. Every synthesis is different and, as we noted before, there are often many synthetic routes that can be designed for any single target molecule.


