7.3: The Wittig Reaction
- Page ID
- 354666
This reaction allows us to synthesize alkenes by adding the two carbons of the alkene double bond together. This is a very powerful synthetic technique that allows us to construct larger molecules from smaller ones. It is much more efficient to add two molecules together, than to try and synthesize a large molecule one carbon-carbon bond at a time.
The Wittig reaction starts by preparation of a reagent that involves addition of an alkyl halide to a phosphine (the phosphorus analog of an amine), such as triphenylphosphine (\(\mathrm{Ph}_{3}\mathrm{P}\)). This reaction is directly analogous to the alkylation of amines that we have seen previously to produce the phosphonium salt as shown. However, the reaction can go further in the presence of a base to produce the stabilized carbanion by deprotonation of the carbon next to the phosphorus as shown[11].[12]
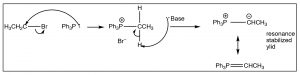
The Wittig reagent can then react with an aldehyde or ketone as shown. The first step, as we might imagine, involves nucleophilic attack at the carbonyl, but since the carbanion is also attached to the phosphorus, another avenue of reaction is now available. The tetrahedral intermediate undergoes further reaction in which the oxygen bonds to the phosphorus and then, via a cyclic rearrangement of electrons, the four-membered ring rearranges to form the alkene and triphenylphosphine oxide. In fact, it is the formation of the very strong \(\mathrm{P=O}\) double bond that makes this reaction essentially irreversible, and drives the reaction towards products.
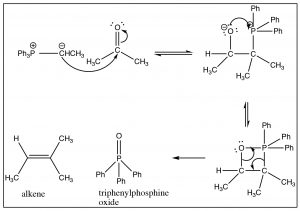
The Wittig reaction can be used to prepare many different alkenes. In general, if there is a possibility of E/Z isomerism, the most stable alkene will be produced (for example a trans isomer rather than a cis isomer). But, just as we have seen many times, it is possible to manipulate the reactants and reaction conditions to produce the desired product—a subject for a more advanced treatment of organic chemistry[13].


