3.2: Optical Isomerism
- Page ID
- 354349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
In the previous section we looked at conformational and geometric stereoisomerism, by which we mean that molecules that have the same molecular formula and connectivity of the atoms, but that have different arrangements in space. Conformational isomers can be interconverted by rotations around \(\mathrm{C-C}\) bonds while geometric (cis/trans) isomers have permanently distinct geometries. Now we introduce another type of stereoisomer, known as optical isomers. Optical isomers have a property called chirality—they are mirror images of one another. While they have exactly the same overall shape, they are not superimposable on one another. One obvious example of macroscopic objects that are chiral are your hands.

Your hands are mirror images of each other, but they are not superimposable on one another placing one hand on top of the other shows you that they are different as does the fact that a left hand glove does not fit your right hand. There are many objects in the world that are chiral. At the molecular level, many molecules are chiral—and it is often said that they exist in left- and right-handed forms.
The simplest examples of chiral molecules are those that have a single carbon atom with four different substituents attached. While these compounds have exactly the same structure, connectivity and relationship between the atoms in space, they are different in the same way that a right and left hand are different: they are known as enantiomers—non-superimposable mirror images of each other. An equal mixture of enantiomers is called a racemate (racemic). Under normal laboratory conditions it is difficult to differentiate between a pair of enantiomers. If you were able to separate the left- from the right-handed forms, you would find that they have the same structure, the same physical and chemical properties, that is, the same melting point, boiling point, solubility, and chemical reactivity. Nevertheless, they are not identical—they can be distinguished by the way they interact with certain types of light.
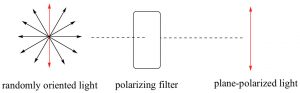
Electromagnetic radiation can be considered as being composed of electrical and magnetic (e-m) waves that oscillate perpendicular to each other and to the direction of wave transmission.
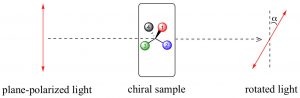
But a beam of light is not a single e-m wave; it consists of many such waves. The orientation of the various e-m waves within the beam are independent on one another and oriented randomly with respect to the direction of the beam. We can simplify an e-m wave by passing the light beam through what is known as a polarizer. A polarizer is a transparent material (it allows light to pass through it) composed of oriented crystalline that block all e-m oscillations that do not align with the crystal structure. The result is that the light that passes through the polarizer oscillates in only one plane; known as plane-polarized light. You can now make a prediction; if you place a second polarizing filter in the same orientation as the first, in the path of a plane-polarized light beam, the beam will pass through, but if you rotate the second filter by \(90^{\circ}\) to the first, the beam will be absorbed (blocked). This is an experiment you can do if you have polarizing sunglasses, which reduce glare by allowing light vibrating in only one direction to pass through. If the plane-polarized light interacts with an asymmetric electric field (such as the one generated by a chiral molecule), the plane of vibration will be rotated. We can measure the angle of rotation using another polarizing lens and in this way, we can detect the presence of chiral molecules. On the other hand, passing plane-polarized light through a racemic mixture (with 50% of each enantiomers present), or through a solution containing a non-chiral solute, will not affect the direction of the polarization plane.
In a sample that rotates the plane of a plane-polarized light beam to the right, the enantiomer is designated as the (+ or dextrorotatory ) isomer; if it rotates the plane-polarized light beam to the left it is referred to as the (– or levorotatory ) isomer. This phenomenon is direct evidence of the existence of chiral molecules. The amount of rotation is dependent on the concentration of the substance in the solution, the path length of the sample tube, the wavelength of the incident light, and also the nature of the substance itself. We often report these rotations as a specific rotation, where all of these variables are defined, so that compounds can be identified by this property. Because of this phenomenon, substances that are chiral are said to be optically active.


