3.1: Conformations of Organic Molecules
- Page ID
- 354276
While most \(\mathrm{C-C}\) single bonds do allow for free rotation, there are energy costs that are associated with such rotations which arise from the fact that as the carbons rotate around the bond axis, the distance between groups attached to each carbon (even if they are hydrogens) changes. To understand the implications of this changing distance between these groups, recall our previous discussions of London dispersion forces and van der Waals interactions.[2] As two uncharged atoms, molecules, or groups within a molecule approach each other there is initially an attraction between them due to the instantaneous and induced dipoles in their electron clouds (London dispersion forces). If the molecules (or parts of a molecule) also have a permanent dipole (i.e. the molecule is polar), then there is an additional attractive (or repulsive) interaction in the form of dipole-dipole interactions and hydrogen bonding. Attractive interactions lead to a decrease in the potential energy of the system and repulsive interactions lead to an increase. Even when the interaction is attractive, as the interacting molecules (or regions of molecules) get closer, the repulsive interactions between their electron clouds increase and the potential energy of the system increases. This gives rise to the familiar (or at least it should be familiar) potential energy v distance curve that we have encountered many times in CLUE.
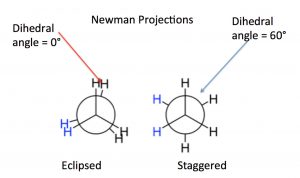
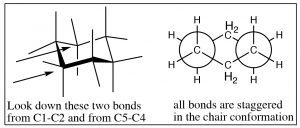
While we have previously discussed this change in potential energy for separate molecules (and atoms), the same principles apply as parts of molecules approach each other, for example, because of rotations around \(\mathrm{C-C}\) sigma bonds. The different rotational structures of a molecule are called conformations, and one of the best ways to represent different possible conformations is called the Newman projection. In a Newman representation, we look down the \(\mathrm{C-C}\) bond of interest; this has the effect of making the groups (atoms, etc.) attached to each carbon (known as substituents) appear to be at angles of \(120^{\circ}\) to each other (this is due to the perspective from which we are viewing the molecule; the bond angles are still \(109^{\circ}\)). If we imagine the carbons rotating with respect to one another, we can see how the relative distances between the groups on each carbon change with the “dihedral angle” between the substituents on the front and back carbons. The two conformations shown for ethane are the fully eclipsed conformation, where the dihedral angle is equal to \(0^{\circ}\), and the staggered conformation, where the dihedral angle is \(60^{\circ}\). As the rotation around the \(\mathrm{C-C}\) bond continues, these two conformations repeat.
Based on NMR studies at low temperature, these two conformations are not equally stable, the staggered conformation is about \(13.8 \mathrm{~kJ} / \mathrm{mol}\) more stable than the eclipsed conformation. At room temperature, this energy difference is negligible, that is, a typical molecular collision supplies much more energy, and the two conformations rapidly interconvert between one another. As the sample is cooled down, however, the energy available from collisions is reduced (because the molecules are moving more slowly) and we can actually find, based on NMR studies, that we can see different conformations present in different proportions—in part because in the eclipsed conformation the hydrogens on the two carbons come closer to their van der Waals radii and so begin to repel one another, raising the potential energy of the molecule.
Potential Energy v Dihedral Angle for Butane
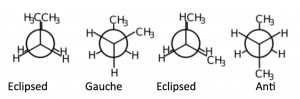
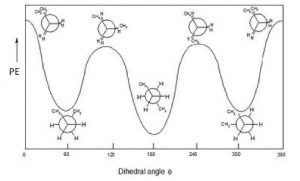
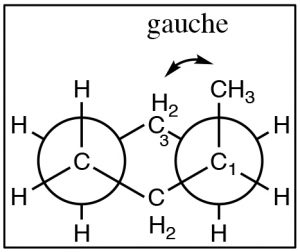
If we look at butane (\(\rightarrow\)) down the \(\mathrm{C}2\mathrm{-C} 3\) bond axis we can see a more exaggerated version of this repulsion due to the presence of the bulkier methyl groups. There are two types of eclipsed conformations, and two types of staggered conformations—the anti conformation where the methyl groups are \(180^{\circ}\) from each other, and the gauche conformation where they are \(60^{\circ}\) apart. If we plot their relative energies vs dihedral angle we can see how the potential energy changes as the larger centers of electron density (the methyl groups) get closer to one another. The anti conformation is the most stable and the eclipsed methyl group conformation is the least stable. Again, these energy differences are not all that large. The difference between the highest-energy eclipsed conformation and the lowest-energy anti conformation is about \(21 \mathrm{~kJ} / \mathrm{mol}\), which is not a big enough barrier to prevent rotation at room temperature.
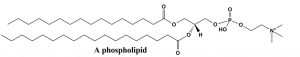
By applying these ideas, we can predict conformations of long-chain alkanes as well. We usually draw long-chained alkanes in the most stable (all-“anti”) conformation, but there are occasions when the gauche conformation is also present—the result of this conformation is a bend or kink in the chain. This has implications how long-chained alkyl groups interact with one another, and influences the molecules’ physical properties, such as melting point. Many biologically-significant lipid molecules contain long-chain alkane groups. For example phospholipids form the structural basis of biological membranes. These lipids are a major component of the cell membrane that is the permeable barrier that surrounds the cell. The long-chain fatty acids that make up the membrane are typically found in the all-anti conformation, which is not only the lowest-energy conformation, but it also makes for the largest surface area. If the long-chains were in the gauche conformation, they would be more clumped up and have a lower surface area. Having a large surface area means that the chains are more likely to attract each other by London dispersion forces, which also stabilizes the structure of the membrane.
Questions to Answer
- Construct an explanation for why the potential energy of ethane changes as you rotate around the \(\mathrm{C-C}\) bond. What is the interaction that is raising the potential energy? Where does the energy come from—so that the rotation around the \(\mathrm{C-C}\) bond can occur?
- Construct a potential energy v dihedral angle diagram for propane, as you look down the \(\mathrm{C}1\mathrm{-C} 2\) bond. Label the maxima and minima with the corresponding Newman projections.
- Draw a schematic picture of a lipid bilayer made up of phospholipids (no need to draw every bond and atom). What would be the effect if the \(\mathrm{C-C}\) bonds in phospholipids took up the less stable gauche conformation?
Conformations of Cyclic Compounds
As we will see, cyclic compounds show some of the same kinds of energy changes with rotations around \(\mathrm{C-C}\) bonds; conformations change from eclipsed to staggered. However, when the molecule is cyclic two other factors come into play and they complicate things a little. The first is that, by their very nature, ring compounds are more constrained; it is not possible to do a full \(360^{\circ}\) rotation around the \(\mathrm{C-C}\) bonds without breaking a covalent bond (which requires more energy than is available through thermal collisions). There is, however, a range of rotations that most ring compounds can move through. The other factor involves rings that would have bond angles that are inconsistent with the \(\sim 109^{\circ}\) bond angle that is usually found for \(\mathrm{sp}^{3}\) hybridized carbons.
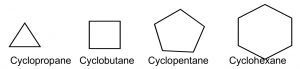
For example, consider the \(\mathrm{C-}3\) to \(\mathrm{C-}6\) cycloalkanes (\(\rightarrow\)). We can calculate the bond angles for these regular polyhedrons if they were flat, that is, two-dimensional: they would range from \(60^{\circ}\) for cyclopropane to \(120^{\circ}\) for cyclohexane, both far outside the range of \(\mathrm{sp}^{3}\) bond angles. Not surprisingly, cycloalkanes behave in a range of different ways to minimize the strain imposed by both bond angles and the constraints on bond rotation. This ring strain can be experimentally determined by measuring the heats of combustion for cycloalkanes: the energy released by this reaction is a proxy for the stability of the particular cycloalkane. To compare among cycloalkanes, we need to calculate the heat of combustion per \(\mathrm{CH}_{2}\) group, and as we will see shortly, six-membered rings do not, in fact, have any ring strain so we can determine the stabilities of differently sized rings compared to cyclohexane.
|
Cycloalkane |
Heat of combustion(\(\mathrm{kJ} / \mathrm{mol}\)) |
Heat of combustion per \(\mathrm{CH}_{2}\) (\(\mathrm{kJ} / \mathrm{mol}\)) |
Ring Strain Compared to \(\mathrm{C}_{6} \mathrm{H}_{12}\) (\(\mathrm{kJ} / \mathrm{mol}\)) |
Total Ring Strain \(= \text { Ring strain } \times \# \text { of } \mathrm{CH}_{2}\) (\(\mathrm{kJ} / \mathrm{mol}\)) |
|
C3H6 |
-499.8 |
-166.3 |
9.2 |
27.6 |
|
C4H8 |
-655.9 |
-164 |
6.6 |
26.4 |
|
C5H10 |
-793.5 |
-158.7 |
1.3 |
6.5 |
|
C6H12 |
-944.5 |
-157.4 |
0 |
0 |
Heats of Combustion for the Cycloalkanes
Cyclopropane is actually the worst-case scenario: it has the highest strain because it is forced to exist as a flat, \(2\mathrm{-D}\) ring (since three points define a plane). If we look at a Newman projection of cyclohexane we can see that all the \(\mathrm{C-H}\) and \(\mathrm{C-C}\) bonds are eclipsed, which raises the potential energy of the molecule.
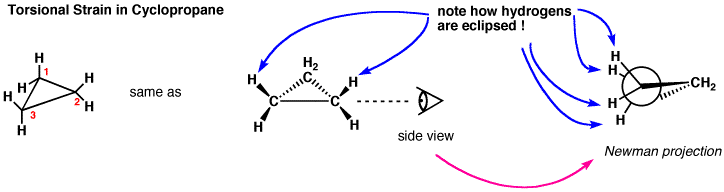
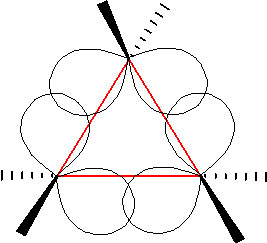
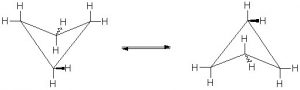
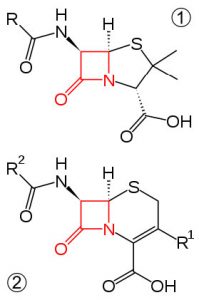
In an attempt to relieve some of the strain imposed by having a \(60^{\circ}\) bond angle, cyclopropane has bent bonds (sometimes called banana bonds). The electron density bulges out rather than being located between the two carbons, thus making the bond angle a little larger—however, there is still considerable strain. The combination of angle and eclipsing strain (also called torsional strain) explains why cyclopropane is a much more reactive species than its straight-chain analog, propane. There are a few naturally occurring three-membered ring compounds, but in general they are quite chemically reactive since the ring structure is quite unstable. Four-membered rings are more stable—they have less angle strain because the ring is bigger and because the larger ring can bend a little, which has the effect of relieving some of the torsional strain. At room temperature, the cyclobutane ring is constantly bending so that each carbon can be relieved of a little of the strain. There are a number of important four-membered ring compounds that you may have run across: the antibiotics penicillin (1) and cephalosporin (2) have a four-membered amide ring as part of their structure (à). Normally (as we will see), amide groups are relatively stable (chemically nonreactive), but because this amide in the ring structure is constrained in a high-energy, four-membered ring, it is much more reactive than normal. Antibiotics act by targeting some part of the biochemistry of bacteria that will not affect the host organism (us). These antibiotics interfere with the synthesis and replication of the microbial cell wall, which is a type structure that most animals do not have (we have cell membranes).
Ring stability improves with cyclopentane. If the molecule were flat, it would have almost exactly the \(\mathrm{sp}^{3}\) bond angles between the ring carbons; this would mean, however, that all the bonds would be in the eclipsed conformation and the resulting torsional energy would be high. To relieve torsional strain the molecule bends into a sequence of “envelope” type shapes—again in dynamic and rapid motion so that the energy associated with the eclipsed conformation is reduced.
This brings us to cyclohexane, the “goldilocks” of cycloalkanes. The bending of the cyclohexane molecule can completely relieve all angle strain and torsional strain. In fact, cyclohexane is as stable as hexane—there is no ring strain associated with a six-membered ring. The most stable conformation of cyclohexane is known as the chair conformation, because one might imagine it as a chair with a footrest, seat, and back. In the chair conformation all the bonds are staggered, and all bond angles are \(109^{\circ}\).
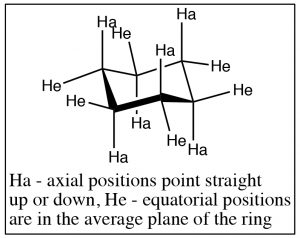
The groups attached to the cyclohexane ring can assume two types of positions: the positions that seem to point straight up or down are called axial, and the ones that are more in the plane of the ring are called equatorial. On each carbon, there is one axial and one equatorial position as shown (\(\uparrow\)).

Chair conformations can “ring-flip” as shown below. Here the structure on the left has all the axial positions in blue and the equatorial in red. Just as with the other rings, the ring is in constant motion and the bonds are rotating (as far as they can within the confines of the ring) so that the ring will “flip” back and forth between chair conformations, by bringing the chair back down and the footrest up. When this happens, axial substituents are converted to equatorial and vice versa. It’s worth noting that axial components can be “above” or “below” the ring plane; and the equatorial positions are also above or below the plane—but on average they lie in the plane of the ring. The other thing to notice is that while groups can flip between axial and equatorial, they do not flip from above to below. That is an axial substituent above the plane becomes and equatorial substituent above the plane.
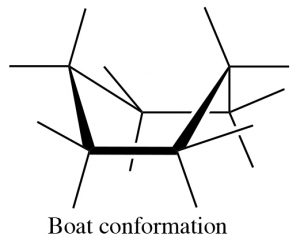
There are a number of conformations between one chair conformation and another, for example the boat conformation (\(\rightarrow\)). This is a much more high-energy species since many of the bonds are eclipsed and there is a strong repulsion of the H’s at the 1 and 4 position.
Mono-substituted cyclohexanes:
Now consider methylcyclohexane, the methyl group can be either axial or equatorial. When we look at Newman projections we see that the equatorial methyl group is in a more stable position, it is anti to the \(\mathrm{C-C}\) bond in the ring, whereas an axial group is gauche to the ring \(\mathrm{C-C}\) bond.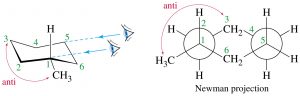
Equatorial methylcyclohexane
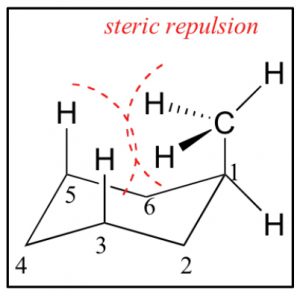
In addition, an axial methyl group is also close to the axial hydrogens on carbons 3 and 5 in what is called a 1.3 diaxial interaction—which introduces another source of strain. Therefore, the more stable conformation is always equatorial.
Axial methylcyclohexane
For a methyl group the difference in energy is still quite small, and both axial and equatorial are present at room temperature, but as bulkier groups are introduced onto the ring, the preference for equatorial becomes even stronger. For example, cyclohexane rings with isopropyl (\(-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)) or t-butyl (\(-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)) groups are locked into conformations where these groups are equatorial.
Disubstitutedcyclohexanes:
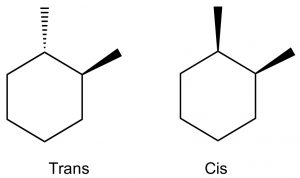
When we get to cyclohexane rings that have two (or more) substituents, we need to consider whether the substituents are on the same (cis) or opposite (trans) sides of the ring. This is easiest to see if we first draw the ring flat and use a wedge dash representation to show relative positions of the groups. In these representations we don’t know whether the methyl groups oriented in axial or equatorial configurations (but we can figure it out), but what we do know is that these are different compounds, they cannot be interconverted by ring-flips like axial and equatorial—which are conformations of the same compound. To interconvert we would have to break the \(\mathrm{C-C}\) bonds in the ring. These two compounds are called geometric isomers—they have the same molecular formula and connectivity but their atoms have a different (permanent) relationship in space (a different geometry).
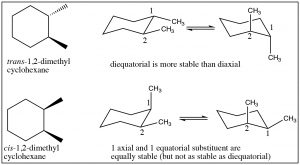
We can determine the actual conformations that each isomer can take up by drawing the chair representation, and we can ring-flip to find the most stable conformation. Remember that equatorial is always favored, so when we do this we can see that the trans isomer has a conformation in which both methyl groups are equatorial; whereas the cis conformation has both conformations where one is axial and one is equatorial. Therefore, we can conclude that the trans isomer is more stable than the cis. Note that the groups do not change their cis or trans relationship when the ring-flips.
It is possible to do this kind of analysis for 1,3- and 1,4-disubstituted cyclohexanes, and even for more complex substances. For example, most sugars actually exist as ring structures in which the many alcohol groups (which are bigger than hydrogen) take up the equatorial position. For example, glucose exists as cyclic six-membered rings (one of the atoms is an oxygen but the principle is the same).
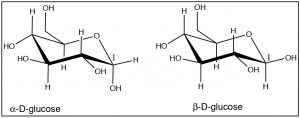
Note how almost all of the \(\mathrm{OH}\) groups take up the equatorial position, except for the \(\mathrm{OH}\) group on \(\mathrm{C-}1\) in \(\alpha\)-D-glucose. These two forms of glucose are actually separate compounds. It is not possible to convert \(\alpha\)-D-glucose to \(\beta\)-D-Glucose simply by a ring-flip (convince yourself that this is true by making model). We will see much more of these cyclic sugar molecules later in the course.
Questions to Answer
- Use excel to plot total ring strain vs ring size (number of \(\mathrm{CH}_{2}\) units) as shown in Table 1. In addition use these total ring strain values for \(\mathrm{C}_{7} \mathrm{H}_{14}\) (\(6.3 \mathrm{~kJ} / \mathrm{mol})\)), and \(\mathrm{C}_{8} \mathrm{H}_{16}\) (\(9.6 \mathrm{~kJ} / \mathrm{mol})\)), \(\mathrm{C}_{9} \mathrm{H}_{18}\) (\(12.6 \mathrm{~kJ} / \mathrm{mol})\)). What trends do you see? How do you account for them?
- Draw two chair forms of cyclohexanol, which one is most stable? Is this a geometric isomer or a conformational isomer.
- Draw chair forms of cis and trans-1,3-dimethylcyclohexane and predict which geometric isomer is the most stable. Do the same for cis and trans-1,4-dimethylcyclohexane.
- Does 1,5-dimethylcyclohexane exist?


