1.3: Effect of pH on Acid Base Reactions
- Page ID
- 354158
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)So far, we have discussed situations when the acid or base is added to solution of pure water. Pure water has a \(\mathrm{pH}\) of 7, and \(\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\left[{ }^{-} \mathrm{OH}\right]\). Now let us consider what happens when we change the \(\mathrm{pH}\) of the solution. For example consider a situation in which we dissolve a simple organic acid (\(\mathrm{CH}_{3}\mathrm{CO}_{2}\mathrm{H}\), acetic acid) in a solution that has a \(\mathrm{pH}>7\), that is where the \(\left[{ }^{-} \mathrm{OH}\right]>\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\); under these conditions the extent of the acetic acid’s ionization is increased. Recall that in 1M acetic acid only \(\sim 3%\) of the acid is ionized at \(\mathrm{pH } 7\). If we change the solution to make it basic by adding \(\mathrm{NaOH}\), the excess of strong base (\({ }^{-} \mathrm{OH}\)) will completely deprotonate the acid. At equilibrium, the reaction will now favor products over reactants (i.e. it will move to the right). What we have done here is drive the acetic acid \(\leftrightarrows\) acetate reaction to the right, increasing the concentration of acetate, which is an application of Le Chatelier’s principle). Note that \(\mathrm{Na}^{+}\), derived from the addition of the \(\mathrm{NaOH}\) used to adjust the \(\mathrm{pH}\), is present but does not take part in the reaction – for this reason it referred to as a “spectator ion”. Another, perhaps simpler, way to predict the outcome of this reaction is to use the \(\mathrm{pK}_{\mathrm{a}}\) values of the two acids (\(\mathrm{CH}_{3} \mathrm{CO}_{2} \mathrm{H}, 4.8\) and \(\mathrm{H}_{2} \mathrm{O}, 14\)), clearly acetic acid is a much stronger acid than water, and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. What we have done here is change the acetic acid, which is a polar organic molecule, into acetate, an ionic species.

Acetic acid is a small organic molecule; since it is polar it can interact with water (though intermolecular forces), therefore acetic acid is very soluble in water (indeed it is miscible with water (it has unlimited solubility.)[9] But now, let us consider the effect of increasing the length of the hydrocarbon group of the organic acid on its molecular properties. Acetic acid has a methyl (\(\mathrm{CH}_{3}-\)) group, the smallest possible hydrocarbon. In contrast dodecanoic (lauric) acid has a 12-carbon hydrocarbon chain (\(\mathrm{CH}_{3}\left[\mathrm{CH}_{2}\right] 11 -\)) and has a solubility in water of \(0.063 \mathrm{g} / \mathrm{L}(\sim 30 \mathrm{mM})\) at \(25^{\circ} \mathrm{C}\), which is much less that of acetic acid.[10] As the hydrocarbon (non-polar) part of the molecule increases in length, solubility in water decreases: the \(\Delta \mathrm{G}\) of the process of dissolving the organic acid in water becomes more positive. This decrease in solubility is primarily due to a negative entropy change (\(\Delta \mathrm{S}\)) caused by the self-organization of water molecules around the hydrocarbon “tail” of the molecule. Now let us consider the behavior of ionized sodium dodecanoate (the sodium salt of dodecanoic acid); it, like many ionic species, it is soluble in water. Although as you may remember, this is a different form solubility – the soluble species is not isolated molecules but rather molecular complexes known as micelles (\(\rightarrow\)).[11] The upshot of this is we can “solubilize” organic acids in water by deprotonating them, but if we then lower the pH, the organic acid will separate from solution again.

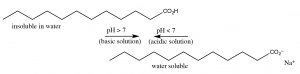
Organic bases can be solubilized in a similar way, except that now the solution must be made acidic. For example, a nitrogenous base with a large non-polar group such as dodecylamine (\(\mathrm{C}_{12} \mathrm{H}_{27} \mathrm{~N}\)) has a solubility of about \(3.5 \mathrm{~g} / \mathrm{L}(\sim 20 \mathrm{mM})\), but at acidic \(\mathrm{pH}\)s it is completely soluble. Contrast the solubility of dodecyl amine with cadaverine (\(\mathrm{NH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}\)), the compound that smells like its name, which is completely miscible with water because it has two polar amino groups. It turns out that we can predict the pH at which a particular acid or base protonates or deprotonates. You may recall from general chemistry that the pH of weak acids and their conjugate bases (like most organic species) can be described using the Henderson Hasselbalch equation (\(rightarrow\)).
One way to work with this equation is to note that \([\text { acid }]=\left[\text { conjugate } p H=p K_{a}+\log \frac{[\text { base }]}{[\text { acid }]} \text { base }\right]\) when the \(\mathrm{pH}\) of the system is equal to \(\mathrm{pK}_{\mathrm{a}}\) of the acid. At \(\mathrm{pH}\)’s below \(\mathrm{pK}_{\mathrm{a}}\), \(\text { [acid] }>\text { [conjugate base] }\), there is more acid than base, and vice versa for \(\mathrm{pH}>\mathrm{pK}_{\mathrm{a}}\). Therefore, by adjusting the \(\mathrm{pH}\) we can change the concentrations of conjugate acid and base to suit our purposes, or we can predict the relative concentrations at any \(\mathrm{pH}\). For example, acetic acid with a \(\mathrm{pK}_{\mathrm{a}}\) of \(4.8\) would have \(50 \% \mathrm{CH}_{3} \mathrm{CO}_{2} \mathrm{H}\), and \(50 \% \mathrm{CH}_{3} \mathrm{CO}_{2}^{-} \mathrm{Na}^{+}\), at a \(\mathrm{pH}\) of \(4.8\). If the \(\mathrm{pH}\) falls below \(4.8\) the concentration of protonated acid will increase, and if it rises the concentration of acetate ion will increase.
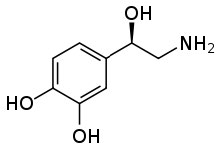
This ability to transform an organic substance from an insoluble (in water) molecule to a soluble ionic species can be very useful. One common example stems from the fact that many pharmaceutical drugs are organic substances that are insoluble in aqueous solutions (like cytoplasm or blood). If these substances were introduced into the body in their non-ionized form they would not dissolve, and therefore be inactive. If you check the labels on many prescription bottles you will see that the drug is administered as a salt. Consider norepinephrine (\(rightarrow), a hormone that is often administered intravenously to counteract the effects of allergic reactions. It is administered as a salt of tartaric acid to ensure that it is soluble in the blood stream.
You may come across another example of this phenomenon (that acids are soluble in basic solution, and bases are soluble in acid solution) if you take the organic chemistry laboratory course. If your product has an acidic or basic moiety in its structure, you can extract the substance into aqueous (acid or basic) solution, washing away all the organic by-products with an organic solvent, and then regenerating the acidic or basic substance. This is an important purification method for many substances, because it allows the compound of interest to be separated into aqueous solution and then regenerated simply by adding or subtracting a proton.

When we consider biomolecules (that is, organic molecules found in organisms) the situation is not so clear cut; most biomolecules have a variety of acidic and basic groups as part of their structure. Even the simplest amino acid, glycine (\(rightarrow\)) exist in a variety of protonated and deprotonated forms depending on the \(\mathrm{pH}\).
One thing that becomes clear is that individual amino acids are always charged regardless of the \(\mathrm{pH}\), so they are water-soluble. But the extent of the protonation/deprotonation reactions is \(\mathrm{pH}\) dependent. As we will see this has a number of ramifications for a wide range of biological molecules, because they will behave very differently in different pH solutions. This is one important reason why most biological systems are buffered so that they remain at a fairly constant \(\mathrm{pH}\).
Questions to Answer:
- If you have a mixture of benzoic acid \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\left(\mathrm{pK}_{\mathrm{a}}\right. \text { 4.2) }\), toluene, \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}\) and aniline hydrochloride (\(\mathrm{pK}_{\mathrm{a}}\) of \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{3}{ }^{+} 4.6\)). Which substance will be soluble in aqueous acidic solution, which will be soluble in aqueous basic solution, which will not be soluble in water?
- Outline a scheme for separating these three substances by using their differing solubilities in organic and aqueous solutions of different \(\mathrm{pH}\)s.


