1.2: Acid-Base Reaction Direction and Position of Equilibrium
- Page ID
- 354157
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Acid base reactions begin because of electrostatic interactions, but the extent to which the reaction proceeds depends on the relative Gibbs free energy of the reactants and products, that is, the overall Gibbs free energy change (\(\Delta \mathrm{G}\)) for the reaction. This is a subtle but important point: the reaction does not occur because the products are more stable, it occurs because there is an attractive force between two reactants that have polar structures, As we will see, we can predict the relative amounts of reactants and products in a mixture (at equilibrium), based both on an understanding of molecular structures and by comparing their \(\mathrm{pK}_{\mathrm{a}}\).
Acid Strength (using the Brønsted–Lowry model):
The strength of an acid, that is the degree to which it donates \(\mathrm{H}^{+}\) to (or accepts electron pairs from) other molecules, depends on a number of factors including, obviously, the strength of the base (that is the degree to which the base donates electron pairs to other molecules) it reacts with. Acid and base strengths are usually reported using water as the solvent (i.e. as the base or acid respectively), so that acid strengths can be compared directly. Since biological reactions take place in aqueous solution we will be able to extend our understanding of simple acid base reactions to much more complex ones as we move forward.
The reaction for any acid HA is: \[\mathrm{HA}+\mathrm{H}_{2} \mathrm{O} \rightleftarrows \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{A}^{-}\]
We can estimate the extent of the reaction (i.e., how far the reaction goes, that is the concentrations of reactants and products when the reaction reaches equilibrium) by determining the equilibrium constant \(\mathrm{K}_{\mathrm{a}}\). \[\mathrm{K}_{\mathrm{a}}=\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{A}^{-}\right] /[\mathrm{HA}]\]
In contrast to strong inorganic acids (such as \(\mathrm{HCl}\), or \(\mathrm{HNO}_{3}\)), the equilibrium constants for many organic acids are small (ranging from 10–1 to 10-55) and it is more common to report \(\mathrm{pK}_{\mathrm{a}}\) – which, as you will remember, is \(=-\log \mathbf{K}_{\mathbf{a}}\). A strong acid such as \(\mathrm{HCl}\) has a large \(\mathrm{K}_{\mathrm{a}}\) (in fact it is so large as to be meaningless) and therefore a very small (negative) \(\mathrm{pK}_{\mathrm{a}}\).
|
Acid |
Ka |
pKa |
|---|---|---|
|
HCl (hydrochloric acid) |
~107 |
–7 |
|
CF3COOH (Trifluoroacetic acid) |
3.2 x 10–1 |
0.5 |
|
HF (hydrofluoric acid) |
7.2 x 10–4 |
3.14 |
|
CH3COOH (acetic acid) |
1.8 x 10–5 |
4.8 |
|
H2O |
10-14 |
14 |
|
CH3CH2OH (acetic acid) |
10-16 |
16 |
| NH4+ (ammonia in NH4Cl) | 5.6 x 10–10 | 9.25 |
| CH4 (methane) | ~10–55 | 55 |
It helps to be able to interpret these numbers in terms of the extent of the associated reaction. For example, water (which acts as both an acid and a base) dissociates to a very small extent. In a liter of pure water, which contains ~54 moles of water molecules (or \(\sim 54 \times 6.02 \times 10^{23}\) molecules or \(\sim 3.25 \times 10^{25}\) molecules), \(\sim 10^{-7}\) moles (or \(\sim 10^{-7} \times 54 \times 6.02 \times 10^{23}\) molecules or \(\sim 3.25 \times 10^{16} \mathrm{H}_{3} \mathrm{O}^{+}\) ions). The weaker the acid the higher the \(\mathrm{pK}_{\mathrm{a}}\) (can you explain why that is the case and what it means in terms of the relative concentrations of species at equilibrium?).
It will help you greatly if you memorize a few important approximate \(\mathrm{pK}_{\mathrm{a}}\) values for common acids, for example alcohols tend to have a \(\mathrm{pK}_{\mathrm{a}}\) of \(\sim 15\), while amines have a \(\mathrm{pK}_{\mathrm{a}} \sim 33\). As we will see the \(\mathrm{pK}_{\mathrm{a}}\) of various carbon species is very dependent on the environment of the \(\mathrm{C-H}\) bond, but remembering that \(\mathrm{sp}^{3}\) carbon-hydrogen bonds (\(\mathrm{pK}_{\mathrm{a}} \sim 55\)) are not likely to ionize under any circumstances is helpful. However, it is even more important to understand the factors that affect acid strength, and be able to use them to predict and explain the outcomes of reactions.
Another important idea to remember is that the extent of a reaction (as measured by its equilibrium constant \(K\)) is related to the change in Gibbs free energy (\(\Delta \mathrm{G}^{\circ}=\Delta \mathrm{H}^{\circ}-\mathrm{T} \Delta \mathrm{S}^{\circ}\)) associated with that reaction. That is when we think about the extent of a reaction (the concentration of reactants and products when the reaction reaches equilibrium) in terms of the relative stabilities of the reactants and products we need to take into account both the enthalpy change (\(\mathrm{H}^{\circ}\)), which reflects the changes in bonding and intermolecular interactions involving both reactants and products, and the entropy change (\(\Delta \mathrm{S}^{\circ}\)) associated with the reaction system. Recall that \(\Delta \mathrm{S}^{\circ}\) reflects change in the number of possible energy states and positions in the reaction system. For most organic (weak) acids, it turns out that the \(\mathrm{H}^{\circ}\) of the dissociation reaction in water is approximately zero, because the types of bonding and interactions that are broken and formed during the reaction are similar. Differences in \(\Delta \mathrm{G}\) for the reaction (and therefor \(\mathrm{K}_{\mathrm{a}}\) and \(\mathrm{pK}_{\mathrm{a}}\)) are typically due to differences in \(\Delta \mathrm{S}\).
Questions to Answer
- Explain why acids and bases are always (as pairs) found together in a system.
- What is meant by the terms conjugate acid or conjugate base?
- In the Lewis model for the \(\mathrm{HCl}\) + water reaction, explain why you draw the arrow pointing from \(\mathrm{O}\) to \(\mathrm{H}\).
- Complete these acid base reactions and predict the relative amounts of reactants and products when the reaction reaches equilibrium for each reaction. Explain your predictions using your knowledge of atomic and molecular structures and electronegativity. \[\begin{aligned}
&\mathrm{CH}_{3} \mathrm{NH}_{2}+\mathrm{HCL} \rightleftarrows \\
&\mathrm{CH}_{3} \mathrm{NH}_{2}+\mathrm{H}_{2} \mathrm{O} \rightleftarrows \\
&\mathrm{CH}_{3} \mathrm{NH}^{-}+\mathrm{H}_{2} \mathrm{O} \rightleftarrows \\
&\mathrm{CH}_{3} \mathrm{NH}_{3}^{+}+\mathrm{H}_{2} \mathrm{O} \rightleftarrows
\end{aligned}\]
Organic Acids and Bases
Having reviewed acids and bases using rather simple molecules (\(\mathrm{HCl}\) and \(\mathrm{NH}_{3}\)), let us move on to the more complex world of organic acids and bases, how to identify them, how to determine relative strengths, and how to predict what will happen in any given mixture. We begin by comparing the \(\mathrm{pK}_{\mathrm{a}}\)‘s of some organic acids. Let us begin with ethanol (\(\mathrm{pK}_{\mathrm{a}} \sim 16\)), a molecule that we typically do not consider to be an acid, and acetic acid (\(\mathrm{pK}_{\mathrm{a }} 4.8\)). There is clearly a huge difference between the \(\mathrm{pK}_{\mathrm{a}}\)’s of these two molecules, the question is can we understand why this is the case?
If we draw out their structures we see that both have (as expected) the acidic hydrogen bonded to the electronegative oxygen. (Make sure you remember why the hydrogens bonded to carbons are not as acidic as those bonded to oxygen). So why the huge difference in \(\mathrm{pK}_{\mathrm{a}}\)‘s? To answer this question we have to remember that the extent of the reaction depends on the relative thermodynamic stability of the products—that is, the system containing the conjugate base of the acid and the hydronium ion. The reactions and conjugate bases of the two are shown here (\(\downarrow\)). Based on their \(\mathrm{pK}_{\mathrm{a}}\) values, we would predict that the ethanol dissociation reaction is rare (few ethoxide ions form) while the acetic acid dissociation reaction is more frequent. However note that even in the case of the acetic acid only about 3% of the acid molecules are dissociated in a 1M solution.

The first step in both reactions appears to be more or less the same, an electron pair from the oxygen in water forms a bond to the electron deficient hydrogen while the \(\mathrm{O-H}\) bond of the acid breaks and the electrons originally associated with it the move back to the oxygen. The difference between the two reactions lies mainly in the way that the negatively charged conjugate bases (ethoxide and acetate) behave, and the way that they are solvated by the solvent (water). For ethoxide (ethanol’s conjugate base), the extra negative charge is localized onto the oxygen, which leads to a concentration of charge. Water molecules are strongly attracted to the ethoxide anion, an interaction that limits the mobility of the Resonance Structures Resonance Hybrid water molecules and results in a decrease in entropy (\(\Delta \mathrm{S}\) is negative). In contrast, in acetate (acetic acid’s conjugate base), the negative charge is delocalized onto both oxygens (even though it is often drawn as if it was associated with one but not the other). We can illustrate this in two ways (or more!) by drawing arrows to indicate how the extra electron pair can move from one oxygen to the other; it looks like this (\(\rightarrow\)).

The actual structure has a partial negative charge on both oxygens. This pair of structures is often referred to as a resonance structure and the process is termed resonance but the name is misleading. In fact the actual structure, the resonance hybrid, does NOT involve the electrons (and the double bond) moving back and forth between the two oxygen atoms. By a biological (and not completely sensical) analogy we might say it is a mule or a hinny—the offspring of a cross between a horse and a donkey.[6] Just as a mule (or a hinny) is not bouncing back and forth between being a horse and being a donkey, so the resonance hybrid actually exists as a new species[7], with an actual structure that is partway between the two (drawn) resonance structures. In this case, we are using two bonding models (a valence bond and a delocalized molecular orbital model) to describe the structure of acetate anion. The localized valence bond model involves a sigma single bond framework that connects the atoms and provides the molecular shape. The delocalized molecular orbital model describes a pi bond that connects both \(\mathrm{O}\)s to the \(\mathrm{C}\). We can visualize the anion as a planar \(\mathrm{sp}^{2}\) hybridized carbon connected to a methyl group and two oxygens by sigma bonds together with a 3 atom two electron pi bond that extends over the \(\mathrm{O-C-O}\) framework (\(\rightarrow\)). The result is that in the acetate ion the negative charge is delocalized over two oxygens, rather than being concentrated on only one atom as it is in the ethoxide ion. The result is that the interactions of the acetate with solvent water molecules is not as strong, so that the water molecules are not as ordered, meaning that the water is not as ordered around the molecule and the entropy change is not as negative. The effects of delocalizing charge over more than one atom play a major role in predicting the outcomes of a wide range of reactions. We note that \(\Delta \mathrm{S}\) is still negative since the creation of a charged species still leads to increased ordering of solvent molecules.
One way to predict whether charge can be delocalized is to determine whether resonance structures can be drawn for the charged species. For example: try convincing yourself that you cannot draw resonance structures for ethanol.
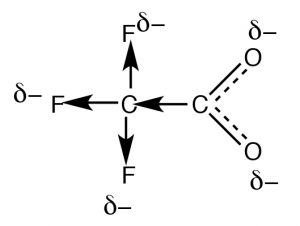
Resonance is not the only way to stabilize charge. Typically, resonance occurs through aconjugated pi bond system, such as occurs within the \(-\mathrm{CO}_{2}^{-}\) part of an organic acid, but how do we account for the difference in the acidities of acetic acid (\(\mathrm{pK}_{\mathrm{a }} 4.8\)) and trifluoroacetic acid (\(\rightarrow) (\(\mathrm{pK}_{\mathrm{a }} 0.5\)), even though they both have the carboxylate functional group? The difference between the two lies in the fact that the charge on the trifluoroacetate anion is delocalized by two distinct mechanisms. As in acetate, the negative charge is delocalized by resonance through the pi bonding system; in addition it is also delocalized onto the fluorines by the fact that the highly electronegative fluorine atoms (more electronegative than \(\mathrm{O}\)) withdraw electrons from the methyl carbon through the sigma bonds, which in turn withdraws electrons from the next carbon, and in turn from the two oxygens (a process known as “induction”). The result is that the negative charge is “smeared out” over even more atoms, making the anion even less likely to cause a solvent molecule ordering (reducing the effect on \(\Delta \mathrm{S}\)). As you might expect, the inductive effect is distance dependent (perhaps you can predict the effect of adding more \(\mathrm{CH}_{2}\) groups between the \(\mathrm{CF}_{3}\) and \(\mathrm{CO}_{2}\) groups).
Questions to Answer:
- Using resonance structures predict which is more acidic: \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}\) or \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}\)?
- Draw structures to show how sodium ethoxide and sodium acetate are solvated in water, and use them to show why the negative entropy change for the formation of sodium acetate is smaller than that of sodium ethoxide.
- Consider the \(\mathrm{pK}_{\mathrm{a}}\)‘s of the three chlorobutanoic acids: \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CHClCOOH}\left(\mathrm{pK}_{\mathrm{a}}\right. \text { 2.86) }\), \(\mathrm{CH}_{3} \mathrm{CHClCH}_{2} \mathrm{COOH}\left(\mathrm{pK}_{\mathrm{a}}\right. \text { 4.05) }\), and \(\mathrm{CH}_{2} \mathrm{ClCH}_{2} \mathrm{CH}_{2} \mathrm{COOH}\left(\mathrm{pK}_{\mathrm{a}}\right. \text { 4.53) }\). Draw structures and use them to explain why these carboxylic acids have different \(\mathrm{pK}_{\mathrm{a}}\)‘s.
Organic Bases
As noted previously, there are no acids without bases, and vice versa. Even if we are only discussing \(\mathrm{H}^{+}\) (proton) transfer, it is (arguably) easier to think about the base using a Lewis model. That is, a base has an electron pair available for donation into a bond with the acid. Recall that almost everything that has a pair of non-bonding electrons (sometimes called a lone pair) can act as a base. The most common types of organic bases often have a nitrogen atom somewhere in their structure. If we compare the basicity of \(\mathrm{N}\), \(\mathrm{O}\) and \(\mathrm{F}\), each of which have lone pairs that are could potentially be donated, nitrogen is the least electronegative and therefore the best able to donate its electrons into a bond, since its lone pair is least attracted by the nucleus. Fluorine, the most electronegative element, holds its electrons very close to the nucleus, and under normal circumstances would not be considered as a base.
Oxygen, since it is more electronegative than nitrogen is not as strong a base, therefore when ammonia and water are mixed, the only reaction that occurs (and that to a relatively small extent) is a proton transfer from water to ammonia. \[\mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O} \leftrightarrows \mathrm{NH}_{4}^{+}+{ }^{-} \mathrm{OH}\]
The equilibrium constant for this reaction is \(1.8 \times 10^{-5}\) (most of the species in the mixture at equilibrium are reactants)
*insert image here*
Here are some organic bases (\(\rightarrow\)). Note that they are components of a wide range of biologically active molecules, including DNA, hormones and pharmaceuticals. As we will see the basic nitrogen provides an important way to understand the reactivity of a particular species.

For now, however, let us start with a simpler base such as methylamine (\(\mathrm{CH}_{3} \mathrm{NH}_{2}\)) the simplest nitrogenous organic base. Methylamine reacts with acids (\(\downarrow\)) in much the same way that ammonia does; it will react with a strong acid like \(\mathrm{HCl}(\mathrm{aq})\) to produce methylammonium chloride.
Recall that the position of equilibrium can be predicted by comparing the strength (\(\mathrm{pK}_{\mathrm{a}}\)’s) of the two acids. \(\mathrm{HCl}\left(\mathrm{pK}_{\mathrm{a}}-7\right)\) is a much stronger acid than \(\mathrm{CH}_{3} \mathrm{NH}_{3}{ }^{+}\left(\mathrm{pK}_{\mathrm{a}} \sim 10\right)\) and therefore we predict that the equilibrium of the methylamine + \(\mathrm{HCl}\) reaction will lie well to the right. Now consider the reaction in which methylamine reacts with acetic acid (\(\downarrow\)).

Again we can predict the position of equilibrium by comparing \(\mathrm{pK}_{\mathrm{a}}\)’s of the conjugate acids (acetic acid \(4.8\) and \(\mathrm{CH}_{3} \mathrm{NH}_{3}{ }^{+} \sim 10\)). Notice that you can predict the structure of the products simply by following the flow of electrons. We could change the \(\mathrm{CH}_{3}\) (methyl) groups on either methylamine and acetic acid to a wide range of different groups and still be able to predict the product easily, as long as you recognize that the reaction that takes place is a (simple) proton transfer (acid–base). For example, look at the structure of cocaine (above): can you predict what will happen if it were reacted with acetic acid? What would be the structure of the product?
Molecules that contain both an acid and a base:
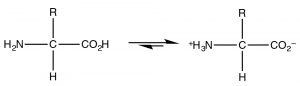
The most common example of a molecule that act as both an acid and a base is of course water because it has both a potentially acidic hydroged, and a lone pair that can accept the proton. However, since this is organic chemistry, where water is not as common a solvent, let us consider the class of molecules that have both acidic and basic domains simultaneously. The most biologically important such molecules are the amino acids, which have both an amino group and a carboxylic acid. A subset of the possible amino acids are those used in biological systems to assemble polypeptides. Amino acids (or rather the \(\alpha\)-amino acids) contain both a carboxylic acid and an amino group attached to a central carbon (the \(\alpha\)-carbon). The generic structure is given here (\(\rightarrow\)) where R stands for a wide range of side chains.[8]At \(\mathrm{pH } 7\) the amino acid exists in what is know as a zwitterionic form, in which the carboxylic acid group is negatively charged while the amino group is positively charged. At no time would an amino acid (dissolved in water) exist in an un-ionized form. We can predict what form would be present at different \(\mathrm{pH}\)’s by considering the \(\mathrm{pK}_{\mathrm{a}}\)‘s of the species involved.