21.4: Chemistry of Acid Halides
- Page ID
- 36401
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- identify the reagent normally used to convert a carboxylic acid to an acid bromide.
- write equations to show how an acid halide may be converted into each of the following: a carboxylic acid, an ester, an amide.
- write a detailed mechanisms for the reaction of an acid halide with each of the following: water, an alcohol, ammonia, a primary or secondary amine.
- identify the product formed when a given acid halide reacts with any of the following reagents: water, an alcohol, a primary or secondary amine.
- identify the acid halide, the reagents, or both, needed to prepare a given carboxylic acid, ester or amide.
- identify the product formed when a given acid halide reacts with water, a given alcohol, ammonia, or a given primary or secondary amine.
- identify lithium aluminum hydride as a reagent for reducing acid halides to primary alcohols, and explain the limited practical value of this reaction.
- identify the partial reduction of an acid halide using lithium tri‑tert‑butoxyaluminum to form an aldehyde.
- write an equation to describe the formation of a tertiary alcohol by the reaction of an acid halide with a Grignard reagent.
- write a detailed mechanism for the reaction of an acid halide with a Grignard reagent.
- identify the product formed from the reaction of a given acid halide with a given Grignard reagent.
- identify the acid halide, the Grignard reagent, or both, needed to prepare a given tertiary alcohol.
- write an equation to illustrate the reaction of an acid halide with a lithium diorganocopper reagent.
- identify the product formed from the reaction of a given acid halide with a given lithium diorganocopper reagent.
- identify the acid halide, the lithium diorganocopper reagent, or both, that must be used to prepare a given ketone.
Acid halides are highly reactive carboxylic acid derivatives. As such they are able to be used to synthesize many other carboxylic acid derivatives.
Formation of Acid Halides
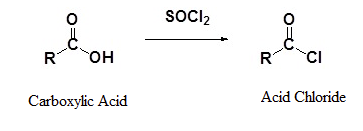
Carboxylic acids react with thionyl chloride (SOCl2) to form acid chlorides. A nucleophilic acyl substitution allows for the replacement of the carboxylic acid –OH with a chloride atom.
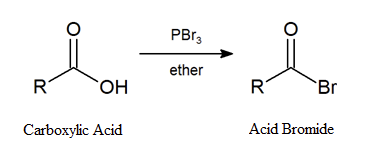
Earlier (Section 10.5), we saw that primary and secondary alcohols react with phosphorous tribromide (PBr3) to afford the corresponding alkyl bromide. In a similar fashion, acid bromides can be formed from the corresponding carboxylic acid by reaction with PBr3.
Reactions of Acid Halides
The high reactivity of acid halides allows them to be easily converted into other acyl compound through nucleophilic acyl substitution. Depending on the nucleophilic reagent applied, acid halides can be used to create carboxylic acids, anhydrides, esters, amides, or ketones. Also, acid halides undergo a double nucleophilic addition with LiAlH4 to produce primary alcohols and Grignard reagents to produce tertiary alcohols. Although this section will only represent reactions with acid chlorides, other acid halides undergo similar reactions.
Conversion of Acid Chlorides to Carboxylic Acids: Hydrolysis
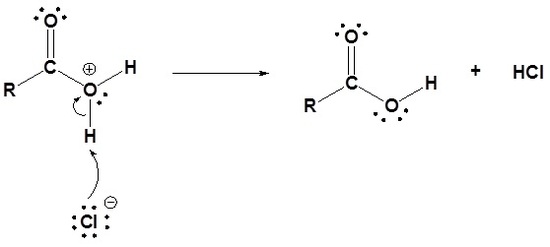
Acid chlorides are converted into carboxylic acids through a nucleophic acyl substitution with water. This reaction follows the typical mechanism where a water nucleophile attacks the electrophilic carbonyl carbon to form a tetrahedral alkoxide intermediate. The carbonyl bond is reformed and Cl- is eliminated as a leaving group. Because water is a neutral nucleophile, an oxonium intermediate in produced. The oxonium intermediate is deprotonated by the chloride anion to produce a neutral carboxylic acid and HCl. The HCl is commonly removed from the reaction mixture by a basic work-up.
General reaction
Example
Mechanism
1) Nucleophilic attack by water
2) Leaving group is removed
3) Deprotonation
Conversion of Acid Chlorides to Anhydrides
Acid chlorides react with carboxylic acids to form anhydrides through a nucleophilic acyl substitution. Because the carboxylic acid nucleophile is neutral, HCl is produced as a side-product during the reaction and is typically removed as part of a basic work-up. Both symmetrical and asymmetrical anhydrides can be created using this reaction. Carboxylates can also be used to form anhydrides in a similar reaction under basic conditions.
General Reaction
Example
Mechanism
1) Nucleophilic attack by a carboxylic acid
2) Leaving group removal
3) Deprotonation
Conversion of Acid Chlorides to Esters: Alcoholysis
Acid chlorides react with alcohol nucleophiles to produce esters. This reaction is the preferred method for preparing esters. Pryidine is often added to the reaction mixture to remove the HCl produced.
General Reaction
Example
Mechanism
1) Nucleophilic attack by an alcohol
2) Leaving group removal
3) Deprotonation
This reaction is particularly affected by steric hindrance so bulky alkyl groups on either the acid chloride or the alcohol significantly decrease the rate. For a given acid chloride there is a reactivity order among alcohols of primary > secondary > tertiary. If a compound has multiple alcohols the less hindered one will be selectively esterified.
Conversion of Acid Chlorides to Aldehydes: Reduction
Acid chlorides can be converted to aldehydes using a hindered reducing agent such as lithium tri-tert-butoxyaluminum hydride LiAlH(Ot-Bu)3 or diisobutylaluminum hydride (DIBALH). These hydride sources are weaker reducing agents than lithium aluminum hydride in part because they are sterically hindered. Also, they have only one equivalent of hydride which makes stoichiometric control of hydride addition much easier.
The mechanism of this reaction is analogous to the hydride reduction of carboxylic acids. We have previously seen that LiAlH4 will reduce carboxylic acids to 1o alcohols thorough an aldehyde intermediate. In that case, the aldehyde intermediate was actually more reactive to hydride reduction than the carboxylic starting material. This prevented the isolation of the aldehyde intermediate because of it quick conversion to the 1o alcohol. LiAlH4 would also convert acid chlorides to 1o alcohols but this reaction would be inefficient because it would be easier to perform the reaction on the corresponding carboxylic acid.
Because acid chlorides are highly activated, they will still react with the weaker hydride sources, to form an aldehyde. Once formed, the aldehyde competes with the remaining acid chloride for the remaining hydride reagent. However, acid chlorides are more reactive towards nucleophilic attack than aldehydes. The acid chloride starting material is quickly consumed by hydride reduction before the aldehyde has a chance to react allowing for isolation of the resulting aldehyde. Using a reaction temperature of -78 oC also helps to isolate the aldehyde as the product by further slowing the aldehyde reduction reaction.
General Reaction
Example
Conversion of Acid chlorides to Amides: Aminolysis
Ammonia, 1o amines, and 2o amines react with acid chlorides to form 1o, 2o, and 3o amides respectively. These reactions typically take place rapidly at room temperature and provides high reaction yields.
The reaction is commonly run with an excess of the amine starting material. Without the excess the amine reactant would eventually become protonated by the HCl produced by the reaction to form a non-nucleophilic ammonium compound. If the amine is not readily available, the reaction is usually run with a base, such as NaOH or pyridine, to neutralize the HCl produced.
General Reaction
Examples
Mechanism
The mechanism of aminolysis follows a typical nucleophilic acyl substitution. The amine nucleophile attacks the carbonyl carbon of the acid chloride forming an alkoxide tetrahedral intermediate. The -Cl leaving group is eliminated, allowing the carbonyl bond to be reformed. Because amines are neutral nuleophiles a protonated amide is produced after this step. In the last step of the mechanism, a second amine acts as a base, removing a proton, and allowing for the amide product to be formed.
1) Nucleophilic attack by an amine
2) Leaving group removal
3) Deprotonation
How could the following molecule be synthsized using an aminolysis of an acid chloride?
Solution
The key bond formed during this reaction is the C-N sigma bond between the carbonyl carbon and the nitrogen. Breaking this bond separated the target molecule into the two required starting materials. The carbonyl carbon gains an –Cl to become an acid chloride and the nitrogen fragment gains an H to become a 1o amine. The acid chloride and the 1o amine can then be joined to form the product.
Conversion of Acid Chlorides to 3o Alcohols: Grignard Reagents
Addition of Grignard reagents converts acid halides to 3o alcohols while forming two C-C bonds. The Grignard reagent adds to the carbonyl carbon twice during this reaction. First, as part of a nucleophilic acyl substitution to form a ketone intermediate. Then as part of a nucleophilic addition to the ketone to form a 3o alcohol.
General Reaction
Predicting the Product of a Grignard Reaction
Example
Draw the products of the following reaction.
Solution
The carbanion nucleophile from the Grignard reagent is added to the carbonyl carbon twice. Once as part of a nucleophilic acyl substitution which eliminates the Cl leaving group. Then again as part of a nucleophilic addition which converts the carbonyl C=O into an alcohol OH. These steps are combined to form a 3o alcohol.
Mechanism
The mechanism starts with the Grignard reagent’s carbanion nucleophile adding to the acid halide carbonyl to form a tetrahedral alkoxide intermediate. The chloride leaving group is then eliminated, reforming the carbonyl to create a ketone intermediate. Once formed, the ketone is in competition with the acid chloride for the Grignard reagent remaining. Although acid chlorides are more reactive toward nucleophilic addition than ketones, the high reactivity of Grignard reagents makes isolating the ketone intermediate difficult. The reaction mechanism continues with the addition of a second carbanion nucleophile to the ketone to form another tetrahedral alkoxide intermediate. Protonation of the alkoxide as part of an acidic work-up creates the 3o alcohol product.
1) Nucleophilic attack
2) Leaving group removal
3) Nucleophilic attack
4) Protonation
Conversion of Acid Chlorides to Ketones: Gilman Reagents
When acid chlorides are reacted with Grignard reagents the ketone intermediate is difficult to isolate because the addition of a second equivalent of the highly reactive Grignard reagent rapidly occurs. Organocuprates however are significantly less reactive than organolithium and organomagnesium reagents and when an acid chloride is reacted with a diorganocuprate (Gillman) reagent (R2CuLi), a ketone product is produced in excellent yields.
General Reaction
Example
Mechanism
The copper atom in organocuprate reagents radically changes the reaction mechanism for their nucleophilic addition to acid chlorides. Computational studies suggest that the reaction mechanism is more complicated than the typical “addition-elimination” sequence seen in nucleophilic acyl substitutions but rather involves multiple mechanistic steps involving complexation with copper and lithium.
The mechanism starts with an oxidative pi-complex formation between the Cu atom in Gilman reagents and the C=O carbonyl bond in acid chlorides. Next, the chloride atom is activated toward elimination through formation of a Lewis Acid/Base complex with a lithium cation. The subsequent elimination of the –Cl leaving cleaves the C-Cl bond and forms a Cu-C bond creating a triorganocopper(III) intermediate. A ketone product is formed when reductive elimination breaks the CuIII-C bond of the intermediate and forms a C-C bond between the carbonyl carbon and an alkyl group from the organocuprate reagent. During the reduction step, copper gains two electrons forming an alkylcopper (CuR) compound as a side product.
This mechanism, in part, explains the selectivity of organocuprates for acid chlorides. Acid chlorides are promoted for this reaction due to the strong electrostatic interaction between chlorine and the lithium cation present. Most other carbonyls compounds, such as ketones, carboxylic acids, esters, acid anhydrides, or amides lack this Cl-Li interaction and react with organocuprate reagents either very slowly or not at all. Because ketones do not react with organocuprate reagents, they are not subject to further nucleophilic additions and are easily isolated as the product of this reaction.
How could the following molecule be synthesized using a Gilman reagent and an acid chloride?
Solution
The key bond formed during this reaction is the C-C sigma bond between the carbonyl carbon and an alpha carbon. Breaking this bond separated the target molecule into two possible two starting materials. The carbonyl carbon gains an –Cl to become an acid chloride and the alkyl fragment becomes part of a Gilman Reagent R2CuLi. The required alkyl fragment becomes the R group in the Gilman reagent. Remember that the Gilman reagent has contains two of the alkyl fragment. Because ketones have two alpha carbons there should be two possible acid chloride/Gilman reagent combinations to make this molecule.
Pathway 1
Solution 1
Pathway 2
Solution 2
Exercises
Q21.4.1
Draw the mechanism for the following reaction
Q21.4.2
Propose a synthesis of the following molecules from an acid chloride and an amide.
(a)
(b)
(c)
Solutions
S21.4.1
S21.4.2
- Acetyl chloride and dimethylamine
- Benzoyl chloride and ethylamine
- Acetyl chloride and ammonia

